molecular mechanisms of head and neck cancer
Molecular mechanisms of loco-regional recurrence and metastasis
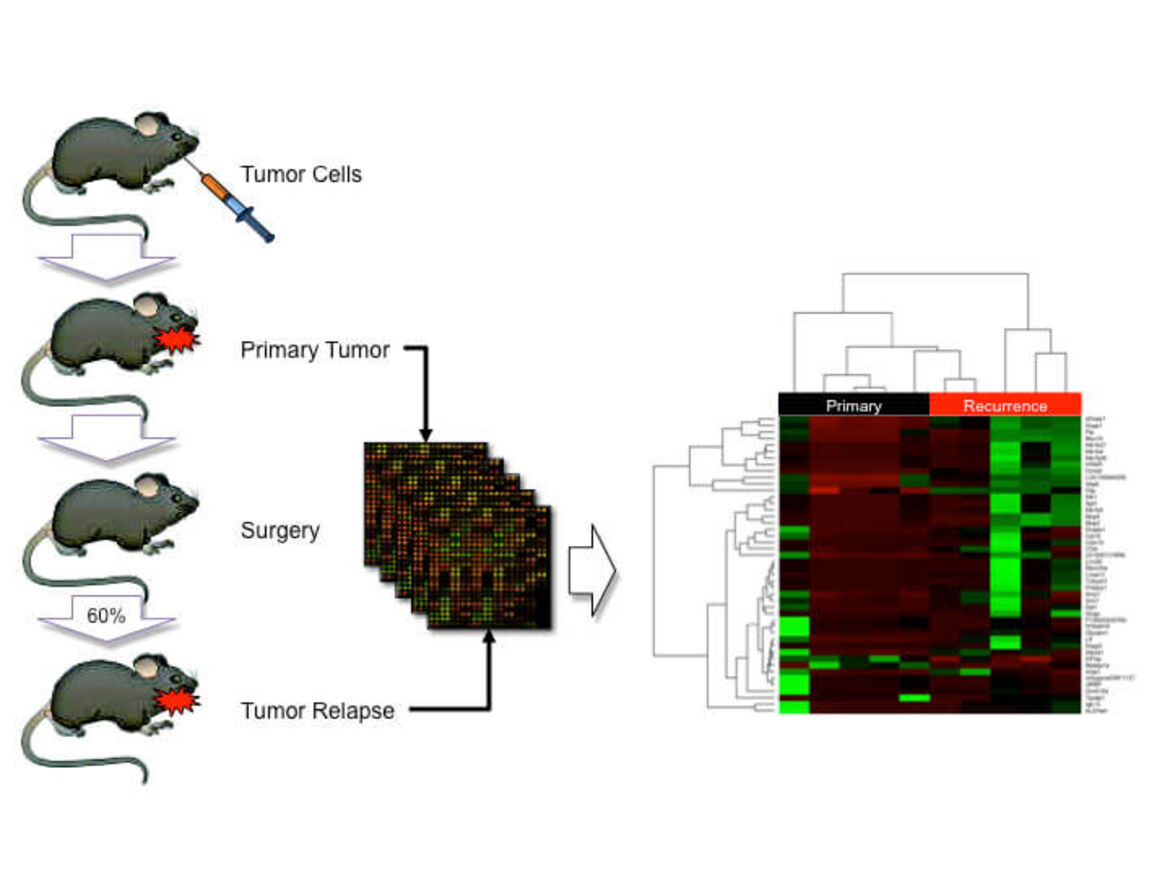
HNSCC is one of the most prevalent and lethal cancers worldwide and mortality mostly results from loco-regional recurrence and metastasis. Despite its significance, our knowledge on molecular, cellular and environmental mechanisms that drive malignant progression remains largely elusive, and there are limited therapeutic options for advanced cancers, with only negligible clinical benefit. Recently, we applied global gene expression profiling with samples derived from an orthotopic floor-of-mouth squamous cell carcinoma model in miceand identified a list of genes with differential expression between primary and recurrent tumors.One differentially expressed gene codes for Myb-binding protein 1a (MYBBP1A), which is known as a transcriptional co-regulator that physically interacts with nuclear transcription factors, such as NFKB and p53. We provided experimental evidence that MYBBP1A is an important molecular switch in the regulation of tumor cell proliferation versus migration in HNSCC and it will be a major challenge for the future to proof the concept whether regulation of MYBBP1A expression and/or function could serve as a novel option for translational oncology.
Proteomic analysis of field cancerization in pharynx and esophagus

‘Field cancerization’ in head and neck squamous cell carcinoma (HNSCC) is poorly understood and it may extend from the pharynx into the oesophagus. Both local recurrences and second primary carcinomas/second field tumors may originate from field cancerization. Recently, we performed a prospective pilot study with the aim to identify patients suffering from field cancerization on the basis of mucosal protein profiles. Five mucosal biopsies from the oropharynx, hypopharynx and from three regions of the esophagus were taken from 24 patients. Protein profiles were generated from the mucosal biopsies. After classifier learning, using the profiles of the patients without tumor diagnosis (n = 9), we were able to discriminate between the different mucosal sites and between healthy mucosa and HNSCC using tumour and healthy tissue samples. Mucosal biopsies of tumor patients (n = 15) revealed changes in the protein profiles similar to those in the tumors. During 42 months median follow-up, six tumor patients experienced local recurrences and second field tumors, of which three occurred in the esophagus. In all six cases, tumor relapse was correctly predicted by altered mucosal protein profiles. Consequently, molecular field cancerization had a strong impact on progression-free survival. Protein profiles of small diagnostic biopsies hold great promise to improve personalized risk assessment in HNSCC.
Selected Publications
Roesch-Ely M, Leipold A, Nees M, Holzinger D, Dietz A, Flechtenmacher C, Wolf T, Zapatka M, and Bosch FX: Proteomic analysis of field cancerization in pharynx and oesophagus: a prospective pilot study. J Pathol 2010; 221: 462–470.
Roesch-Ely M, Schnölzer M, Nees M, Plinkert PK, and Bosch FX: Reference spectra from squamous epithelium and connective tissue allow whole section proteomics analysis. Arch Physiol Biochem 2010 Nov 17. [Epub ahead of print].
S100-RAGE signaling in inflammation and cancer
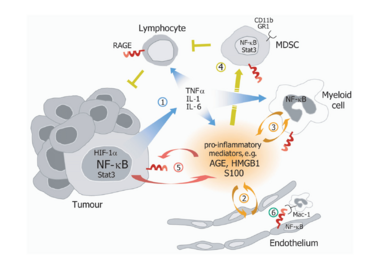
A striking feature of many human cancers is an underlying and unresolved inflammation, which often predates the disease and orchestrates a tumor supporting microenvironment. Indeed, several lines of evidence, including population-based epidemiological and clinical studies as well as experimental animal model systems, highlighted chronic infection and persistent inflammation as major risk factors for various types of cancer. Thus, molecular mechanisms converting a transient inflammatory tissue reaction into a tumor promoting microenvironment as well as signaling and gene regulatory networks implicated in cellular communication between tumor and immune cells will be auspicious targets for innovative strategies of translational cancer research. Recently, we provided genetic evidence that signaling via the receptor for advanced glycation end products (RAGE) drives the strength and maintenance of an inflammatory reaction by converting a transient cellular stimulation into sustained cellular dysfunction during pathological conditions.
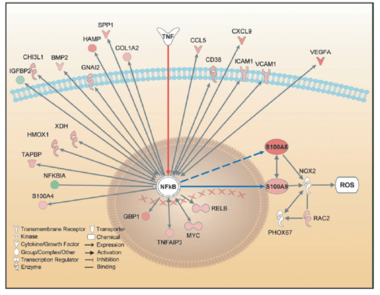
RAGE is a member of the immunoglobulin superfamily of cell surface molecules and interacts with diverse ligands, including not only advanced glycation end products (AGEs) and β-sheet fibrils, but also several members of the S100 protein family (S100B, S100P, S100A4, S100A6, S100A8/9, S100A11–13), high mobility group box-1 (HMGB1), and prions. In the past, we identified an enhanced expression of S100A8 and S100A9 proteins in pathological conditions of chronic inflammation and inflammation-associated carcinogenesis in patient specimens as well as cell culture and experimental animal models. More recently, we identified S100A8 and S100A9 as novel NF-kB target genes in hepatocellular tumor cells during inflammation-associated liver carcinogenesis and provide experimental evidence that increased co-expression of both proteins supports malignant progression by activation of ROS-dependent signaling pathways and protection from cell death.
Selected Publications
Gebhardt C, Nemeth J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol 2006;72:1622-1631.
Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, Hess J, Angel P. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med 2008;205:275-285.
Riehl A, Nemeth J, Angel P, Hess J. The receptor RAGE: Bridging inflammation and cancer. Cell Commun Signal 2009;7:12.
Nemeth J, Stein I, Haag D, Riehl A, Longerich T, Horwitz E, Breuhahn K, Gebhardt C, Schirmacher P, Hahn M, Ben-Neriah Y, Pikarsky E, Angel P, Hess J. S100A8 and S100A9 are novel nuclear factor kappa B target genes during malignant progression of murine and human liver carcinogenesis. Hepatology 2009;50:1251-1262.
Riehl A, Bauer T, Brors B, Busch H, Mark R, Nemeth J, Gebhardt C, Bierhaus A, Nawroth P, Eils R, Konig R, Angel P, Hess J: Identification of the Rage-dependent gene regulatory network in a mouse model of skin inflammation. BMC Genomics 2010, 11:537.