Dengue
International Research Consortium on Dengue Risk Assessment, Management and Surveillance (IDAMS)
| Förderung: | Europäische Union, 7. Rahmenprogramm |
| Laufzeit: | September 2011 – August 2016 |
| WissenschaftlerInnen (Sektion Klinische Tropenmedizin): | T. Jänisch, T. Junghanss, J. Kaur, R. Gaczkowski, K. Rosenberger |
| Kooperationspartner: | · Department of Zoology, University of Oxford, Oxford, UK · Oxford University Clinical Research Unit, Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam · Liverpool School of Tropical Medicine (LSTM), UK · Institute for Research in Biomedicine, Bellinzona, Switzerland · TDR/WHO, Special Program for Research and Training of the World Health Organization, Switzerland · Department of Pediatrics, University of Malaya Medical Center, Kuala Lumpur, Malaysia · Department of Pediatrics, University Gadja Mada, Yogyakarta, Indonesia · Friends Without a Border – Angkor Hospital for Children, Siem Reap, Cambodia · Pedro Kouri Institute of Tropical Medicine, Havana, Cuba · Ministry of Health and Social Welfare – Hospital National de Ninos Benjamin Bloom, San Salvador, El Salvador · Ceara State University, Fortaleza, Brazil · Environmental Research Group Oxford Limited, Oxford, UK · INDEPTH Network, Accra, Ghana · Red Cross / Red Crescent Climate Centre, Den Haag, The Netherlands |
| Web: | www.idams.eu |
Dengue is an emerging disease of major global significance and represents an enormous burden for health care systems in endemic countries. Differentiating dengue from other common febrile illnesses before complications develop is difficult - simple and inexpensive strategies are urgently needed to support early and accurate diagnosis, as well as to identify patients at high risk of developing complications, in order both to improve case management and to facilitate appropriate use of limited resources. Evaluation of early clinical features alongside readily available laboratory tests in a large cohort of patients encompassing the breadth of dengue disease encountered in endemic settings is necessary to develop a robust case definiton for dengue, and could also prove to be very useful for the development of prognostic algorithms. Similarly, characterisation of the profiles of important viral and serological biomarkers in such a cohort is likely to provide valuable information that could contribute additional input into diagnostic and prognostic algorithms. Improved strategies for early diagnosis and risk prediction would also enhance the conduct of clinical trials of early therapeutic interventions for dengue e.g. use of anti-viral or immuno-modulatory drugs. If the fight to gain control of the current global pandemic is to be successful, however, it is equally important to consider interventions and strategies at the population level. Early detection of outbreaks with improved surveillance systems and a prompt effective response to imminent outbreaks, could prove highly significant in reducing the numbers of dengue cases globally. In combination with identification of areas likely to be at risk of dengue outbreaks, as defined by risk mapping, such strategies could bring great health benefits by preventing outbreaks and reducing numbers of cases, with consequent reductions in overall morbidity and mortality from dengue.
For further information please visit our project homepage www.idams.eu
Towards sustainable DENgue COntrol (DENCO)
| Förderung: | Europäische Union, 6. Rahmenprogramm (INCO-DEV) |
| Laufzeit: | 2005 - 2008 |
| WissenschaftlerInnen (Sektion Klinische Tropenmedizin): | T. Jänisch, J. Barniol, T. Junghanss, R. Gaczkowski, K. Rosenberger (Sektion Biostatistik und Epidemiologie) |
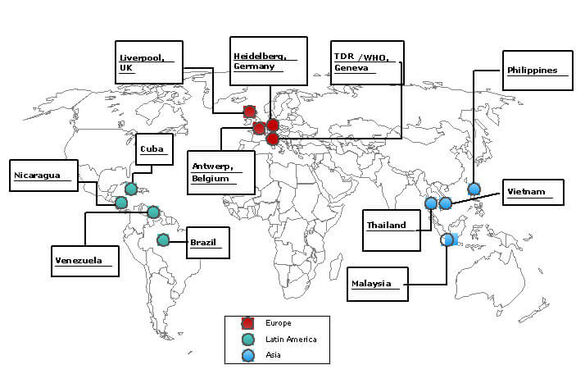
| Kooperationspartner: | ● Dept. of Molecular Virology, University Hospital, Heidelberg, Deutschland ● Liverpool School of Tropical Medicine (LSTM), England ● Prince Leopold Institute of Tropical Medicine (ITG), Antwerp, Belgien ● TDR/WHO, Special Program for Research and Training of the World Health Organization, Schweiz ● Queen Sirikit National Institute of Child Health, Bangkok, Thailand ● Department of Vector-Borne/Blood-Borne Infections, San Lazaro Hospital, Manila, Philippinen ● Oxford University Clinical Research Unit, Hospital for Tropical Diseases and Paediatrics, Ho Chi Minh City, Vietnam ● Department of Paediatrics, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia ● Universidad de los Andes, Research Centre Trujillo, Venezuela ● Virology Department, PAHO/WHO Collaborating Center for Viral Diseases, Tropical Medicine Institute Pedro Kouri (IPK), Havana, Kuba ● Children's Hospital Manuel Jesus de Rivera, Managua, Nicaragua and Department of Virology, National Diagnostics and Reference Center, Ministry of Health, Managua, Nicaragua |
Im DENCO Projekt werden wesentliche Fragestellungen in fünf „work packages“ bearbeitet – von Virologie und Immunologie bis zu Aspekten der Implementierung von Vektor-Kontroll-Maßnahmen.
Die Sektion Klinische Tropenmedizin koordiniert das klinisch-epidemiologische work package mit dem Ziel einer Revision der derzeitigen Dengue Klassifikation in Dengue Fieber (DF), Dengue Hämorrhagischem Fieber (DHF) und Dengue Shock Syndrom (DSS) und – falls möglich – Warnhinweise für einen schweren Krankheitsverlauf zu validieren.
In den letzten Jahren werden bei der derzeit gültigen WHO Klassifikation zunehmend Defizite gesehen. Die WHO Klassifikation stellt die hämorrhagische gegenüber der capillary leakage Komponente als Indikator für eine schwere Verlaufsform in den Vordergrund, was bei Patienten mit einer reinen Schocksymptomatik zu Missklassifikation führt. Ferner gibt es bei der derzeitigen WHO Klassifikation Probleme sowohl bei der Sensitivität (wenn Fälle mit klinischem Schock als Goldstandard für schweren Verlauf betrachtet werden) als auch mit der Spezifität, d.h. dass vom Kliniker mit Standardprotokoll behandelte Fälle unter der derzeit gültigen Klassifikation mit einem Attribut von Schweregrad belegt werden (z.B. DHF - Dengue Hämorrhagisches Fieber).
Das führt dazu, dass auch für pathophysiologische Studien und klinische Studien bessere Endpunkte dringend benötigt werden, die den tatsächlichen Schweregrad der Erkrankung widerspiegeln.
Projektresultate der von der Sektion Klinische Tropenmedizin koordinierten klinischen Projektkomponente: Eine prospektive beobachtende Studie wurde in 11 Krankenhäusern aus 7 Ländern in Südostasien und Lateinamerika durchgeführt. 2259 Kinder und Erwachsene mit Verdacht auf Dengue wurden rekrutiert und mit einem detaillierten Fragebogen-Instrument täglich verfolgt.
Alle Patienten wurden je nach Grad der benötigten medizinischen und pflegerischen Versorgung in 3 Interventionsgruppen eingeordnet. Unter Benutzung eines a-priori erstellten Analyseplans wurden die klinischen und Laborparameter, die diese Interventionsgruppen kennzeichnen, ausgewertet, um letztlich einen empirisch basierten Vorschlag für eine revidierte Klassifikation zu erstellen.