Research Group Chlanda
Research projects in our group focus on "Membrane Biology of Viral Infection".
Projects
We are interested in studying how viruses interact with cellular membranes and lipids during infection. Many enveloped viruses such as influenza or Ebola must cross the cell membrane during entry and exit. To do so, viruses developed or hijacked fascinating protein machinery, which are able to remodel, fuse or cut membranes in processes dependent on specialized lipids. We use and further develop cryo-electron microscopy (cryo-EM) techniques in conjunction with other imaging methods as fluorescence microscopy and imaging mass spectrometry to solve the puzzle of viral-membrane interactions.
Influenza A Virus Entry and Membrane Fusion
Influenza A virus (IAV) is a pleiomorphic, enveloped virus that enters the host cell either by endocytosis or macropinocytosis and fuses with the endosomal membrane in a Hemagglutinin (HA)-mediated process that occurs at low pH. Neither the structure of the HA fusion intermediates nor the spatial orientation of the post-fusion HA with respect to the fusion pore are known. We use cryo-ET and subtomogram averaging to further characterize the structure of HA fusion intermediates. In addition, we study viral fusion and disassembly in the cells close to their native state using cryo-CLEM, cryo-FIB/SEM and cryo-ET. This will allow to further investigate the role of host factors such as aggresome machinery, cytoskeleton, IFITMs proteins and lipids such as cholesterol on the membrane fusion and virion disassembly.
Structual Analysis of Ebola Virus Entry Intermediates
In Vitro and In Situ
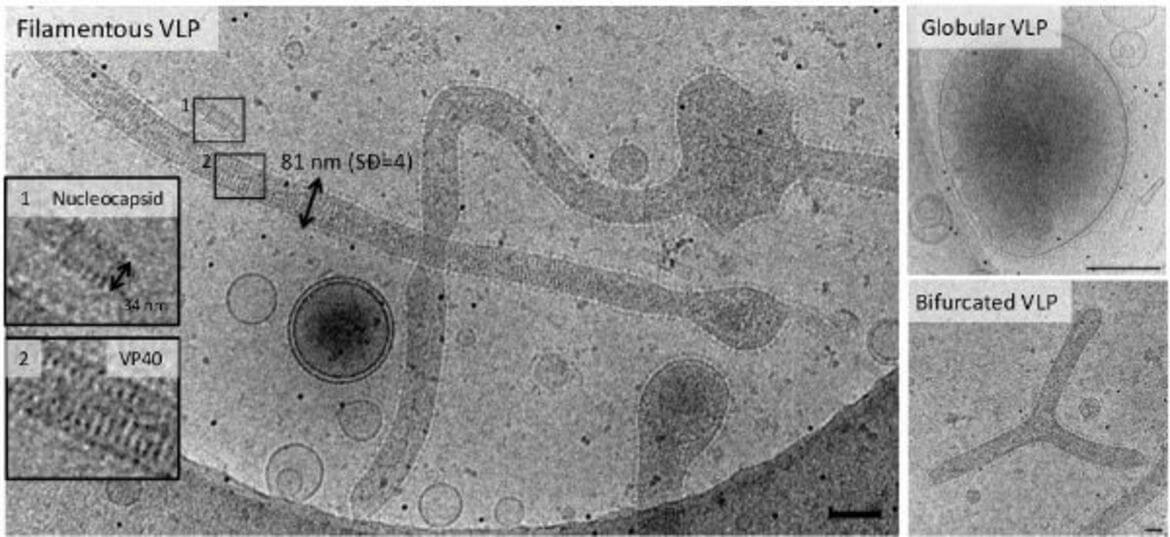
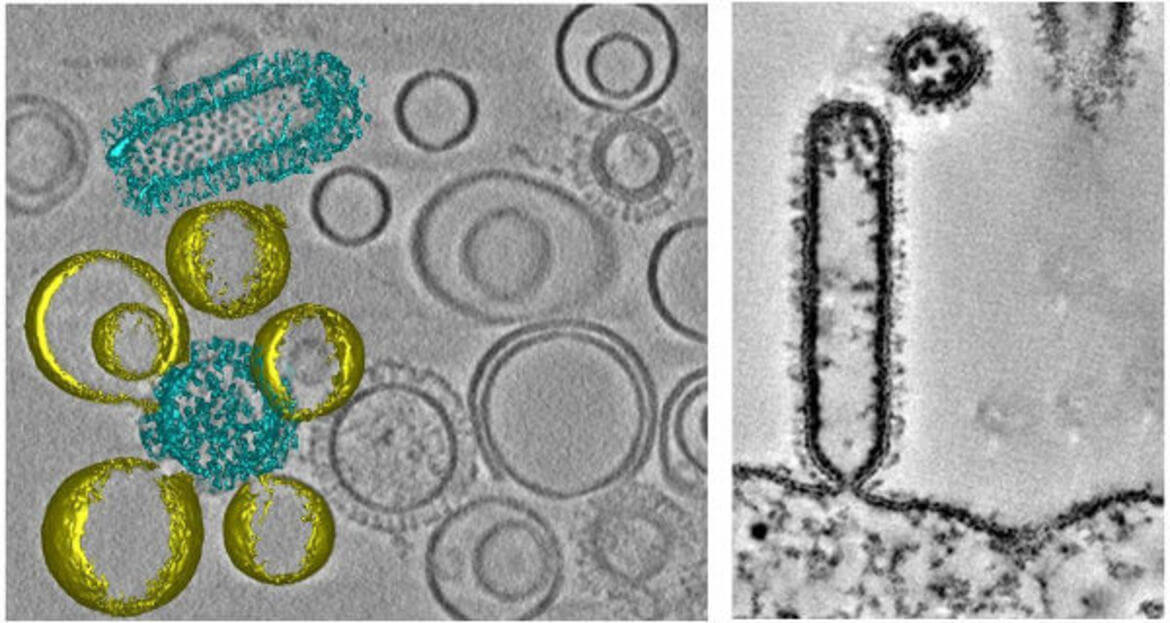
Ebola virus (EBOV) assembles into long filamentous virions (1–15 μm) at the plasma membrane, which upon release, enter epithelial cells by macropinocytosis. The negative single stranded RNA genome is coiled across a length of about 0.9 μm and protected by the nucleocapsid composed of the nucleoprotein (NP), VP35, VP40, and VP24 proteins. The Ebola fusion glycoprotein (GP) is proteolytically processed in the late endosome by low-pH sensitive cathepsin proteases to a 19 kDa fragment, which binds to Niemann-pick-C1 receptor (NPC1). The 19 kDa fragment bound to NPC1 together with yet unknown factor(s) is able to induce membrane fusion allowing the release of the genome. Both the disassembly of the filamentous virus and the mechanism underlying GP mediated membrane fusion in the endosomes are poorly understood processes and have not been structurally characterized. We use non-infectious EBOV VLPs, which are composed of five major structural proteins (GP, NP, VP40, VP35, and VP24) and are structurally similar to EBOV to study EBOV virus entry intermediates by cryo-CLEM, cryo-FIB/SEM and cryo-ET both in vitro and in living cells.
Spatial Lipidomics and Viral Infection
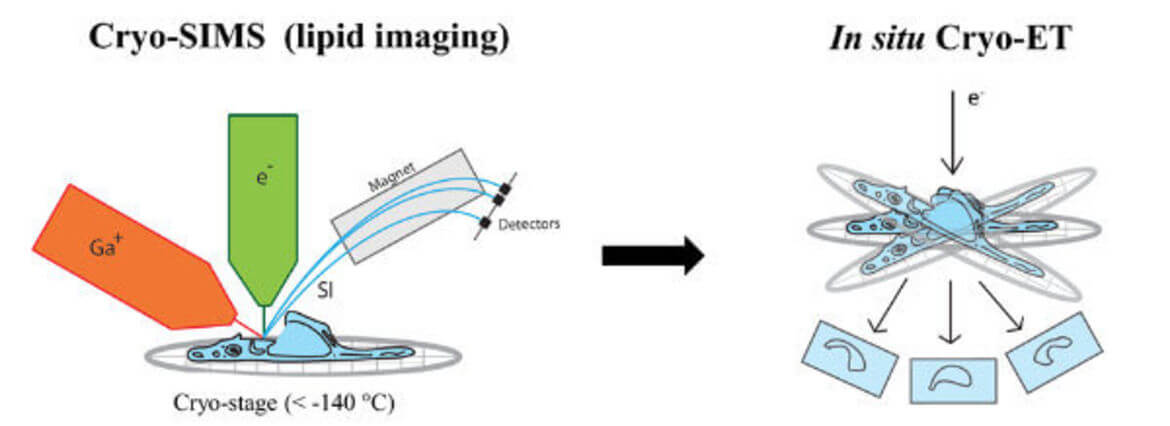
Secondary ion mass spectrometry (SIMS) allows non-invasive imaging of chemically unmodified lipids with high chemical specificity. However, SIMS has so far been only performed on chemically fixed and dehydrated samples (sample preparation procedures known to severely alter membrane structure). In collaboration with Tom Wirtz (Luxembourg Institute of Science and Technology), we will apply SIMS to vitreous cryo-lamellas of the cells prepared by focused-ion beam milling to provide a spatial map of lipids in cellular organelles at native conditions. The lipid map will be subsequently correlated to the membrane structures observed by cryo-ET. We study lipids such as cholesterol which is crucial in host-pathogen interactions as well as in membrane trafficking.
Selected Publications
Complete Publication List (PubMed)
- Chlanda, P., Mekhedov, E., Waters, H., Sodt, A., Schwartz, C., Nair, V., Blank, P.S., Zimmerberg, J. (2017) Palmitoylation contributes to membrane curvature in Influenza A virus assembly and hemagglutinin-mediated membrane fusion. J. Virol. doi: 10.1128/JVI.00947–17.
- Chlanda, P., Krijnse Locker, J., (2017) The sleeping beauty kissed awake: new methods in electron microscopy to study cellular membranes. Biochemical Journal, DOI: 10.1042/BCJ20160990
- Quemin, E.R.J.*, Chlanda, P.*, Sachse, M., Forterre, P., Prangishvili, D. and Krupovic, M., (2016) Eukaryotic-like virus budding in Archaea, MBio, doi: 10.1128/mBio.01439–16. (*Equal contribution)
- Chlanda, P., Mekhedov, E., Waters, H., Schwartz, C.L., Fischer, E.R., Ryham, R.R., Cohen, F.S., Blank, P.S. and Zimmerberg, J. (2016), The hemifusion structure induced by Influenza virus hemagglutinin is determined by physical properties of the target membranes. Nature Microbiology, doi:10.1038/nmicrobiol.2016.50
- Chlanda, P., Zimmerberg, J. (2016), Protein-lipid interactions critical to replication of the influenza A virus during infection. FEBS Lett., doi: 10.1002/1873–3468.12118
- Chlanda, P., Schraidt, O., Kummer, S., Riches, J., Oberwinkler, H., Prinz, S., Kräusslich HG and Briggs, J.A.G. (2015), Structural analysis of the contributions of individual proteins to influenza assembly and morphology. J Virol, 89(17):8957–66.
- Chlanda, P., Sachse, M. (2014), Cryo-Electron Microscopy of Vitreous Sections. Methods Mol Biol, 1117:193–214.
- Krijnse Locker, J., Chlanda, P., Sachsenheimer, T., Brügger, B. (2013), Poxvirus membrane biogenesis: rupture not disruption. Cell Microbiol, 15(2):190–9.
- Chlanda, P., Carbajal, M.A., Cyrklaff, M., Griffiths, G., Krijnse-Locker, J. Membrane rupture generates single open membrane sheets during vaccinia virus assembly. (2009), Cell Host Microbe, 6(1):81–90.
- Cyrklaff, M., Linaroudis, A., Boicu, M., Chlanda, P., Baumeister, W., Griffiths, G., Krijnse-Locker, J. (2007), Whole cell cryo-electron tomography reveals distinct disassembly intermediates of vaccinia virus. PLoS ONE, 2(5):e420.
Group Members
- Dr. Petr Chlanda, Principal Investigator
Petr.Chlanda(at)bioquant.uni-heidelberg.de
BioQuant Room 102a, +49 6221 54-51231
- Steffen Klein, PhD Student
Steffen.Klein(at)bioquant.uni-heidelberg.de
BioQuant Room 103a, +49 6221 54-51230
- Carmen Lahr, M.Sc. Student
C.Lahr(at)stud.uni-heidelberg.de
BioQuant Room 161, +49 6221 54-51251
- Jana Makroczyova, Lab Manager
Jana.Makroczyova(at)bioquant.uni-heidelberg.de
BioQuant Room 102, +49 6221 54-51230
- Anna Michalczyk-Schneider, Administrative Assistant
Anna.Michalczyk-Schneider(at)med.uni-heidelberg.de
INF 324 Room 406, +49 6221 56-32580
- Sarah Peterl, M.Sc. Student
ph249(at)stud.uni-heidelberg.de
BioQuant Room 161, +49 6221 54-51251
- Carl Niklas Schneider, M.Sc. Student
BioQuant Room 161, +49 6221 54-51251
- Melina Vallbracht, Postdoc
Melina.Vallbracht(at)bioquant.uni-heidelberg.de
BioQuant Room 103a, +49 6221 54-51230
- Moritz Wachsmuth-Melm, PhD Student
Wachsmuth-Melm(at)stud.uni-heidelberg.de
BioQuant Room 103, +49 6221 54-51230
- Sophie Winter, PhD Student
Sophie.Winter(at)bioquant.uni-heidelberg.de
BioQuant Room 103a, +49 6221 54-51230
- Liv Zimmermann, PhD Student
Liv.Zimmermann(at)bioquant.uni-heidelberg.de
BioQuant Room 102, +49 6221 54-51230
Alumni
Former Lab Members
- Lisa Augenstein, M.Sc. Student
- Keerthihan Thiyagarajah, M.Sc. Student
- Benedikt Wimmer, M.Sc. Student
Group Leader
For more information about the Chlanda Lab, please check out Website Chlanda Lab
This group is located on the 1st floor of the Center for Quantitative Analysis of Molecular and Cellular Biosystems (BioQuant) at INF 267 and supported by the Chica and Heinz Schaller Foundation Heidelberg