Computational Image Analysis and Medical Physics Team
The Research Team for Computational Image Analysis and Medical Physics is devoted to leveraging sophisticated algorithms and fundamental physics principles to enhance medical diagnostics and interventions. Our commitment involves harnessing cutting-edge computational image analysis tools to extract, quantify, and interpret information from various biomedical imaging modalities, such as CT, MRI, X-ray, electron microscopy, and more.
These state-of-the-art tools play a pivotal role in preclinical research and medical diagnostics by facilitating the detection of anomalies in biomedical images. As technology advances, our approach evolves to incorporate innovative deep-learning techniques into computational image analysis. Our team is dedicated to utilizing deep-learning technologies to analyze large datasets, providing valuable insights into the pathomechanisms of different diseases.
Furthermore, our experienced medical physicists specialize in quantitative CT, including dual-energy and spectral CT, phase-contrast CT, and functional CT imaging methods such as CT perfusion and 4D-CT. Additionally, we conduct intensive research on advanced MRI techniques. Prioritizing radiation safety, our team actively develops strategies to mitigate potential risks, contributing to a safer and more precise approach to medical diagnosis and treatment. Our holistic efforts aim to advance the intersection of computational image analysis, medical physics, and cutting-edge technology for the betterment of healthcare.
Research Projects
Deep Learning-Based Air Trapping Quantification in Children with Cystic Fibrosis using Paired Inspiratory-Expiratory Ultra-Low Dose CT
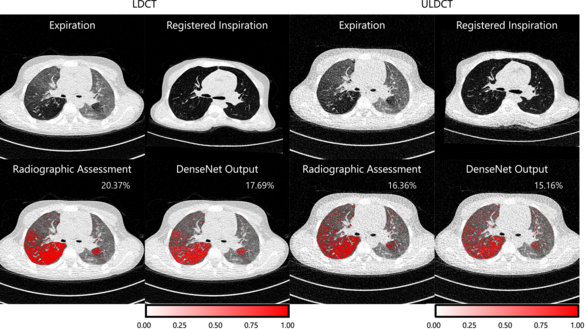
Sarah Muller, Oliver Weinheimer
Cystic fibrosis lung disease is a progressive respiratory condition and air trapping (AT) is one of its important early findings. The correct radiographic assessment of AT on paired inspiratory-expiratory computed tomography (CT) scans is time-consuming and susceptible to inter-reader variation. Conventional methods for AT quantification are density-based and patient-dependent therefore influenced by CT dose and reconstruction. Because of the ongoing trend towards lower radiation doses in lung CT imaging, a continuous revision of quantitative post-processing methods is required. In this study, we investigated a deep learning approach to quantify AT on ultra-low dose in comparison to low dose CT.
Reference: Muller, S.M. et al. (2023). Deep Learning-Based Air Trapping Quantification Using Paired Inspiratory-Expiratory Ultra-low Dose CT. In: Greenspan, H., et al. Medical Image Computing and Computer Assisted
Intervention – MICCAI 2023. MICCAI 2023. Lecture Notes in Computer Science,
vol 14222. Springer, Cham. https://doi.org/10.1007/978-3-031-43898-1_42
Advanced MRI body pulse sequences
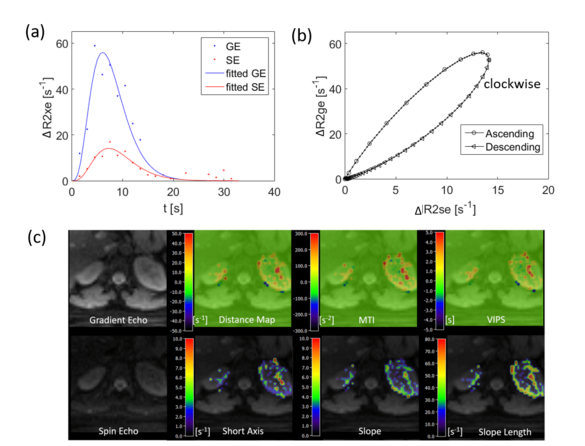
Kidney and liver vessel architecture imaging
Ke Zhang, Simon Triphan, Oliver Sedlaczek, Mark Wielpütz
Vessel architectural imaging (VAI) can be used to measure the structural and topological heterogeneity of microvascular networks. In this study, a navigator-based slice tracking to prospectively compensate the respiratory motion for kidney and liver VAI was developed. The respiratory motion from navigator signal can be precisely calculated and slice positioning can be changed in real-time based on the motion information. The sequence may improve the evaluation of the microvasculature in various kidney and liver diseases.
Reference: https://pubmed.ncbi.nlm.nih.gov/36603781/
Kidney and liver arterial spin labeling
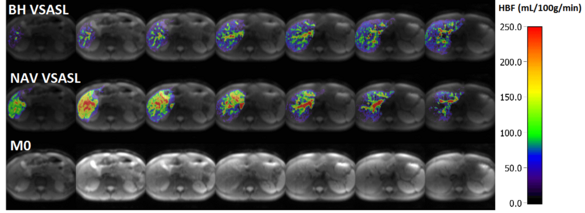
Ke Zhang, Simon Triphan, Oliver Sedlaczek, Mark Wielpütz
The arterial spin labelling (ASL) technique consists in labelling of blood water to obtain quantitative parameters of blood perfusion in the respective tissue; the technique does not require MR contrast agent and is non-invasive. However, to apply the ASL sequence in abdominal imaging, signal noise ratio (SNR) and motion are the major obstacles. To improve SNR velocity selective ASL (VSASL) was applied. To compensate motion, the navigator-based slice tracking was developed.
Reference: https://pubmed.ncbi.nlm.nih.gov/36806110/
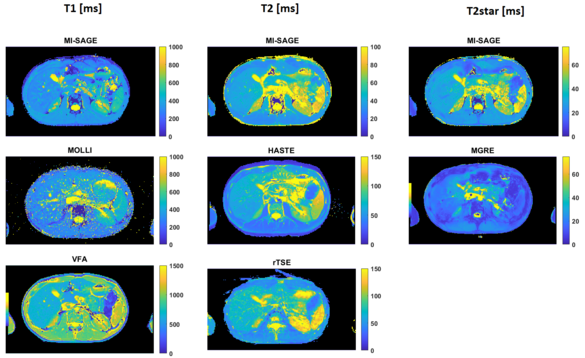
Quantitative T1, T2 and T2* mapping of the liver
Ke Zhang, Simon Triphan, Oliver Sedlaczek, Mark Wielpütz
Quantitative T1, T2 and T2* maps are promising imaging biomarkers for the assessment of liver disease including inflammation, fibrosis, and iron deposition; however these are usually acquired in sequential scans. In this study, a multi inversion spin and gradient echo (MI-SAGE) was developed with ascending slice-order across measurements for simultaenous acquisition with T1, T2, and T2* weighting. The multi-shot variant of the sequence achieved higher in-plane resolution of 1.7 × 1.7 × 8 mm with good image quality in 28s.
Reference: https://pubmed.ncbi.nlm.nih.gov/37939972/
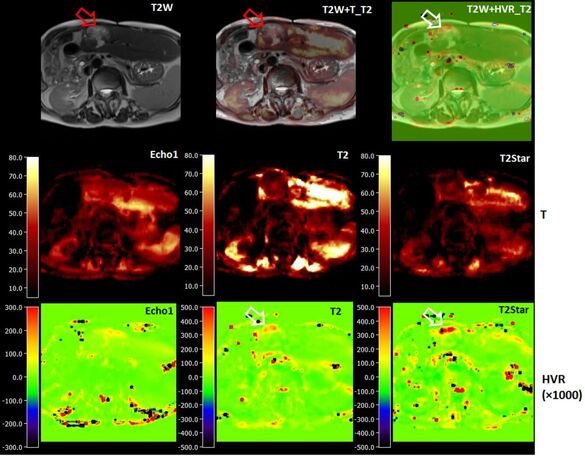
Quantitative liver BOLD
Ke Zhang, Simon Triphan, Oliver Sedlaczek, Mark Wielpütz
MRI has been employed in the assessment of tumor hypoxia to predict treatment outcomes in both preclinical and clinical radiation studies. In BOLD MRI, deoxygenated hemoglobin (deoxy-Hb) serves as an endogenous contrast agent by increasing magnetic susceptibility.
In this study, a navigator-based five-echo spin- and gradient-echo (SAGE) EPI sequence is developed to quantitatively measure T2 and T2* for the purpose of depicting microvascular dysfunction. The sequence is designed to provide adequate signal-to-noise ratio, spatial resolution, and sensitivity to BOLD changes achievable through breath-holding.
Standardization of CT perfusion and development of low radiation exposure CT perfusion alternative for clinical applications in oncologic imaging
Pancreatic dynamic CT perfusion: quantitative meta-analysis
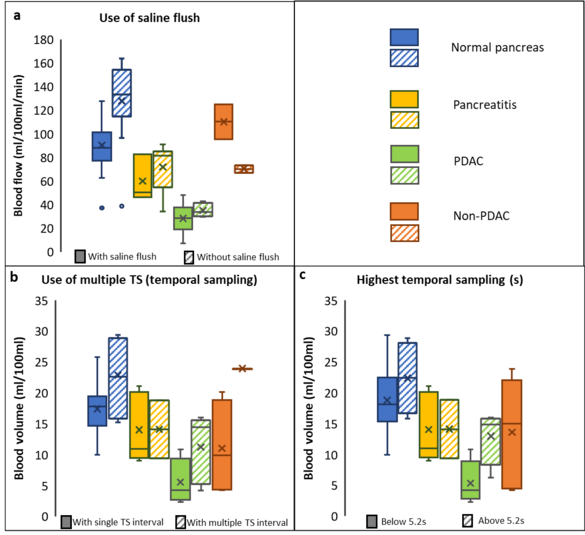
Neha Vats, Stephan Skornitzke, Wolfram Stiller
Dynamic CT perfusion enables measurement of physiological parameters for assessment of diseases that influence tissue perfusion. A number of studies have been performed to find optimum study parameters for CT perfusion measurements, but have also shown changes in the quantitative measurements based on CT examination parameters and evaluation procedures. Thus, a standardization of dynamic CT perfusion acquisition protocols and evaluation procedures is necessary to achieve reliable and clinically meaningful measurements. This study provides a quantitative meta-analysis of pancreatic CT perfusion studies, investigating choice of study parameters, ability for quantitative discrimination of pancreatic diseases, and influence of acquisition and reconstruction parameters on reported perfusion measurements. Furthermore, statistical analysis is performed to confirm the individual study results on the potential applications of perfusion CT as well as to identify the acquisition parameters with the strongest influence on quantitative results for further investigation and standardization of CT perfusion.
Reference: https://pubmed.ncbi.nlm.nih.gov/37477754/
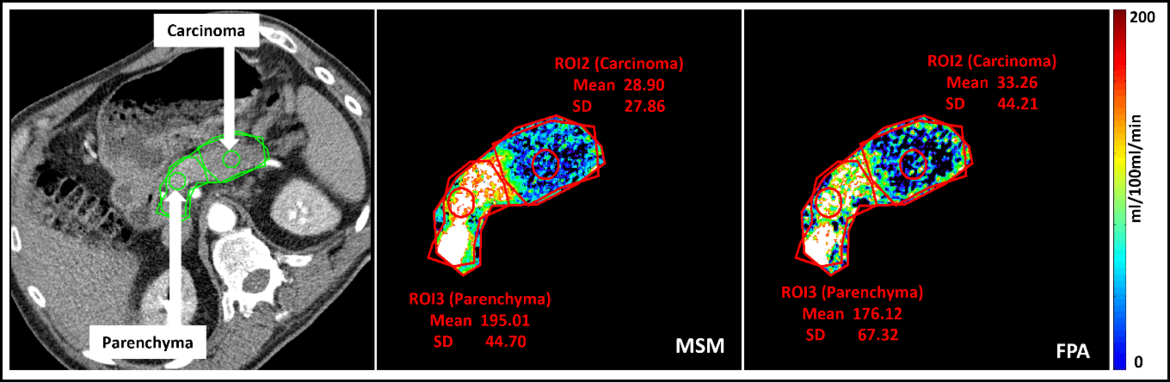
A novel CT perfusion first pass analysis (FPA) technique
Neha Vats, Stephan Skornitzke
Dynamic CT perfusion uses various mathematical models to calculate tissue perfusion measurements that provides useful information for disease diagnosis. It requires multiple volume acquisitions to obtain the perfusion measurements, which leads to a relatively high radiation exposure. On the other hand, the first-pass analysis (FPA) dynamic CT perfusion model uses only two volume acquisitions performed at two time points, thus, potentially drastically reducing the radiation dose by reducing the number of acquisitions. No studies have so far investigated the potential of the FPA technique in the diagnosis of pancreatic diseases. Therefore, the objective of this study was to transfer the FPA technique to the pancreas and to investigate its potential for the accurate detection and diagnosis of pancreatic adenocarcinoma. To this end, the FPA technique was to be validated against MSM and the timing for a clinically applicable acquisition of the two volume scans required by FPA was to be optimized with regard to the pancreas.
Reference: https://pubmed.ncbi.nlm.nih.gov/37391443/
Model-based noise correction of CT perfusion measurements based on digital perfusion phantoms
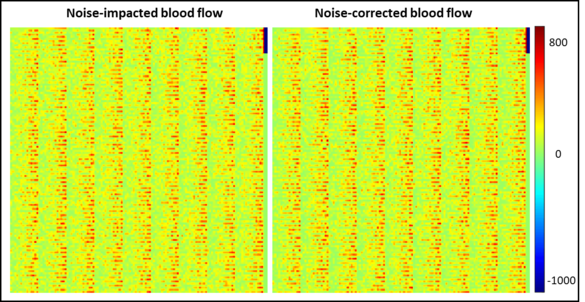
Neha Vats, Stephan Skornitzke
Image noise can negatively influence the accuracy of CT perfusion measurements. To address this issue, as an alternative to studies using clinical image data, where the ground truth of actual blood flow is unknown and measurements are influenced by noise, a noise-correction algorithm has been developed. This algorithm has been implemented with the help of digital perfusion phantoms, allowing the generation of image data from known parameters. With this approach, data can be tailored for each study, avoiding problems of clinical CT perfusion acquisitions, such as radiation exposure to patients, image noise of unknown magnitude, or patient motion. Furthermore, the results obtained using the digital phantoms were applied to retrospectively evaluate the results of an earlier CT perfusion study and provide an estimate of the precision of the measured parameters. With further refinement, algorithm holds the potential to standardize perfusion measurements, enhancing comparability across patients, imaging centres, and equipment vendors, thus contributing to more accurate diagnosis and treatment planning.
Perfusion Quantification in Lung MRI
Detection of cystic pancreatic lesions by MRI in the NAKO study: association with parameters of the metabolic syndrome and pancreatic steatosis
Full automated deep learning scoring for chest X-ray to assess lung disease of cystic fibrosis patients and correlation with lung function testing and chest magnetic resonance imaging
Predicting Mutational Status of Non‐Small Cell Lung Cancer: A Radiomics and Deep Learning Approach
Improving pulmonary embolism detection and risk stratification using deep learning and multimodal data integration
Development of Quantitative Imaging Biomarkers for Abdominal Spectral Computed Tomography
Osteoporotic fracture risk assessment by machine learning-based approach from computed tomography images
Automated Li-Rads classification of liver lesions in gadoxetic acid–enhanced MRI
Dynamic Quantitative Computed Tomography of Ventilation Defects in Muco-Obstructive Lung Disease in Mice
Implementation of phase contrast lung CT for patients at the Italian synchrotron
Deep-learning based noise reduction in computed tomography
Thuy Do, Christopher Schuppert, Tim Weber
AI-based evaluation of sarcopenia in patients with spine metastasis
Thuy Do, Tobias Nonnenmacher
Publications
Selected publications
Triphan SMF, Konietzke M, Biederer J, Eichinger M, Vogelmeier CF, Jörres RA, Kauczor HU, Heußel CP, Jobst BJ, Wielpütz MO; COSYCONET study group. Echo time-dependent observed T1 and quantitative perfusion in chronic obstructive pulmonary disease using magnetic resonance imaging. Front Med (Lausanne). 2024 Jan 5;10:1254003. doi: 10.3389/fmed.2023.1254003. PMID: 38249975; PMCID: PMC10797117
Zhang K, Triphan SMF, Wielpütz MO, Ziener CH, Ladd ME, Schlemmer HP, Kauczor HU, Kurz FT, Sedlaczek O. Simultaneous T1, T2 and T2⁎ mapping of the liver with multi-shot MI-SAGE. Magn Reson Imaging. 2024 Jan;105:75-81.
Almeida SD, Norajitra T, Lüth CT, Wald T, Weru V, Nolden M, Jäger PF, von Stackelberg O, Heußel CP, Weinheimer O, Biederer J, Kauczor HU, Maier-Hein K. Prediction of disease severity in COPD: a deep learning approach for anomaly-based quantitative assessment of chest CT. Eur Radiol. 2023 Dec 27. doi: 10.1007/s00330-023-10540-3. Epub ahead of print. PMID: 38150075
Afsahi AM, Norbash AM, Syed SF, Sedaghat M, Afsahi G, Shahidi R, Tajabadi Z, Bagherzadeh-Fard M, Karami S, Yarahmadi P, Shirdel S, Asgarzadeh A, Baradaran M, Khalaj F, Sadeghsalehi H, Fotouhi M, Habibi MA, Jang H, Alavi A, Sedaghat S. Brain MRI findings in neurologically symptomatic COVID-19 patients: a systematic review and meta-analysis. J Neurol. 2023 Nov;270(11):5131-5154. doi: 10.1007/s00415-023-11914-9.
Athertya JS, Lo J, Chen X, Shin SH, Malhi BS, Jerban S, Ji Y, Sedaghat S, Yoshioka H, Du J, Guma M, Chang EY, Ma Y. High contrast cartilaginous endplate imaging in spine using three dimensional dual-inversion recovery prepared ultrashort echo time (3D DIR-UTE) sequence. Skeletal Radiol. 2023 Nov 8. doi: 10.1007/s00256-023-04503-4.
Khan A, Markus MA, Svetlove A, Hülsmann S, Alves F, Dullin C. Longitudinal x-ray based lung function measurement for monitoring Nintedanib treatment response in a mouse model of lung fibrosis. Sci Rep. 2023 Oct 30;13(1):18637.
Fink MA, Bischoff A, Fink CA, Moll M, Kroschke J, Dulz L, Heußel CP, Kauczor HU, Weber TF. Potential of ChatGPT and GPT-4 for Data Mining of Free-Text CT Reports on Lung Cancer. Radiology. 2023 Sep;308(3):e231362.
Moazamian D, Athertya JS, Dwek S, Lombardi AF, Mohammadi HS, Sedaghat S, Jang H, Ma Y, Chung CB, Du J, Jerban S, Chang EY. Achilles tendon and enthesis assessment using ultrashort echo time magnetic resonance imaging (UTE-MRI) T1 and magnetization transfer (MT) modeling in psoriatic arthritis. NMR Biomed. 2023 Sep 23:e5040. doi: 10.1002/nbm.5040.
Zhang X, Zhou B, Chen Y, Cai Z, Guo Y, Wei Z, Li S, Feng Y, Sedaghat S, Jang. H. Evaluation of gadolinium deposition in cortical bone using three-dimensional ultrashort echo time quantitative susceptibility mapping: A preliminary study. NMR Biomed. 2023 Sep 18:e5035. doi: 10.1002/nbm.5035.
Schierenbeck M, Grözinger M, Reichardt B, Jansen O, Kauczor HU, Campbell GM, Sedaghat S. Detecting Bone Marrow Edema of the Extremities on Spectral Computed Tomography Using a Three-Material Decomposition. Diagnostics (Basel). 2023 Aug 24;13(17):2745. doi: 10.3390/diagnostics13172745.
Muller, S.M. et al. (2023). Deep Learning-Based Air Trapping Quantification Using Paired Inspiratory-Expiratory Ultra-low Dose CT. In: Greenspan, H., et al. Medical Image Computing and Computer Assisted Intervention – MICCAI 2023. MICCAI 2023. Lecture Notes in Computer Science, vol 14222. Springer, Cham.
Bayfield KJ, Weinheimer O, Boyton C, Fitzpatrick R, Middleton A, Kennedy B, Blaxland A, Jayasuriya G, Caplain N, Issa H, Goetti R, Wielpütz MO, Yu L, Galban CJ, Robinson TE, Bartholmai B, Fitzgerald D, Selvadurai H, Robinson PD. Implementation and evaluation of ultra-low dose CT in early cystic fibrosis lung disease. Eur Respir J. 2023 Jul 7;62(1):2300286.
Skornitzke S, Vats N, Mayer P, Kauczor HU, Stiller W. Pancreatic CT perfusion: quantitative meta-analysis of disease discrimination, protocol development, and effect of CT parameters. Insights Imaging. 2023 Jul 21;14(1):132.
Zhang K, Triphan SMF, Ziener CH, Jende JME, Kauczor HU, Schlemmer HP, Sedlaczek O, Kurz FT. Navigator-based slice tracking for kidney pCASL using spin-echo EPI acquisition. Magn Reson Med. 2023 Jul;90(1):231-239.
Backhauß JC, Jansen O, Kauczor HU, Sedaghat S. Fatty Degeneration of the Autochthonous Muscles Is Significantly Associated with Incidental Non-Traumatic Vertebral Body Fractures of the Lower Thoracic Spine in Elderly Patients. J Clin Med. 2023 Jul 8;12(14):4565. doi: 10.3390/jcm12144565.
Sedaghat S, Jang H, Ma Y, Afsahi AM, Reichardt B, Corey-Bloom J, Du J. Clinical evaluation of white matter lesions on 3D inversion recovery ultrashort echo time MRI in multiple sclerosis. Quant Imaging Med Surg. 2023 Jul 1;13(7):4171-4180. doi: 10.21037/qims-22-1317. Epub 2023 May 4.
Vats N, Mayer P, Kortes F, Klauß M, Grenacher L, Stiller W, Kauczor HU, Skornitzke S. Evaluation and timing optimization of CT perfusion first pass analysis in comparison to maximum slope model in pancreatic adenocarcinoma. Sci Rep. 2023 Jun 30;13(1):10595.
Albers J, Wagner WL, Fiedler MO, Rothermel A, Wünnemann F, Di Lillo F, Dreossi D, Sodini N, Baratella E, Confalonieri M, Arfelli F, Kalenka A, Lotz J, Biederer J, Wielpütz MO, Kauczor HU, Alves F, Tromba G, Dullin C. High resolution propagation-based lung imaging at clinically relevant X-ray dose levels. Sci Rep. 2023 Mar 23;13(1):4788.
Sedaghat S, Jang H, Athertya JS, Groezinger M, Corey-Bloom J, Du J. The signal intensity variation of multiple sclerosis (MS) lesions on magnetic resonance imaging (MRI) as a potential biomarker for patients' disability: A feasibility study. Front Neurosci. 2023 Mar 13;17:1145251. doi: 10.3389/fnins.2023.1145251.
Skornitzke S, Vats N, Kopytova T, Tong EWY, Hofbauer T, Weber TF, Rehnitz C, von Stackelberg O, Maier-Hein K, Stiller W, Biederer J, Kauczor HU, Heußel CP, Wielpütz M, Palm V. Asynchronous calibration of quantitative computed tomography bone mineral density assessment for opportunistic osteoporosis screening: phantom-based validation and parameter influence evaluation. Sci Rep. 2022 Dec 1;12(1):20729.
Palm V, Norajitra T, von Stackelberg O, Heussel CP, Skornitzke S, Weinheimer O, Kopytova T, Klein A, Almeida SD, Baumgartner M, Bounias D, Scherer J, Kades K, Gao H, Jäger P, Nolden M, Tong E, Eckl K, Nattenmüller J, Nonnenmacher T, Naas O, Reuter J, Bischoff A, Kroschke J, Rengier F, Schlamp K, Debic M, Kauczor HU, Maier-Hein K, Wielpütz MO. AI-Supported Comprehensive Detection and Quantification of Biomarkers of Subclinical Widespread Diseases at Chest CT for Preventive Medicine. Healthcare (Basel). 2022 Oct 29;10(11):2166.
Athertya JS, Ma Y, Masoud Afsahi A, Lombardi AF, Moazamian D, Jerban S, Sedaghat S, Jang H. Accelerated Quantitative 3D UTE-Cones Imaging Using Compressed Sensing. Sensors (Basel). 2022 Oct 1; 22(19):7459. doi: 10.3390/s22197459.
Dullin C, Svetlove A, Zschüntzsch J, Alves F. Simultaneous assessment of lung morphology and respiratory motion in retrospectively gated in-vivo microCT of free breathing anesthetized mice. Sci Rep. 2022 Aug 2;12(1):13299.
Do TD, Skornitzke S, Merle U, Kittel M, Hofbaur S, Melzig C, Kauczor HU, Wielpütz MO, Weinheimer O. COVID-19 pneumonia: Prediction of patient outcome by CT-based quantitative lung parenchyma analysis combined with laboratory parameters. PLoS One. 2022 Jul 29;17(7):e0271787.
Fink MA, Kades K, Bischoff A, Moll M, Schnell M, Küchler M, Köhler G, Sellner J, Heussel CP, Kauczor HU, Schlemmer HP, Maier-Hein K, Weber TF, Kleesiek J. Deep Learning-based Assessment of Oncologic Outcomes from Natural Language Processing of Structured Radiology Reports. Radiol Artif Intell. 2022 Jul 20;4(5):e220055.
Svetlove A, Albers J, Hülsmann S, Markus MA, Zschüntzsch J, Alves F, Dullin C. Non-Invasive Optical Motion Tracking Allows Monitoring of Respiratory Dynamics in Dystrophin-Deficient Mice. Cells. 2022 Mar 7;11(5):918.
Fink MA, Mayer VL, Schneider T, Seibold C, Stiefelhagen R, Kleesiek J, Weber TF, Kauczor HU. CT Angiography Clot Burden Score from Data Mining of Structured Reports for Pulmonary Embolism. Radiology. 2022 Jan;302(1):175-184.
Triphan SMF, Weinheimer O, Gutberlet M, Heußel CP, Vogel-Claussen J, Herth F, Vogelmeier CF, Jörres RA, Kauczor HU, Wielpütz MO, Biederer J, Jobst BJ; COSYCONET Study Group. Echo Time-Dependent Observed Lung T1 in Patients With Chronic Obstructive Pulmonary Disease in Correlation With Quantitative Imaging and Clinical Indices. J Magn Reson Imaging. 2021 Nov;54(5):1562-1571.
Mayer P, Fritz F, Koell M, Skornitzke S, Bergmann F, Gaida MM, Hackert T, Maier-Hein K, Laun FB, Kauczor HU, Grenacher L, Klauß M, Stiller W. Assessment of tissue perfusion of pancreatic cancer as potential imaging biomarker by means of Intravoxel incoherent motion MRI and CT perfusion: correlation with histological microvessel density as ground truth. Cancer Imaging. 2021 Jan 19;21(1):13.
Triphan SMF, Stahl M, Jobst BJ, Sommerburg O, Kauczor HU, Schenk JP, Alrajab A, Eichinger M, Mall MA, Wielpütz MO. Echo Time-Dependence of Observed Lung T1 in Patients With Cystic Fibrosis and Correlation With Clinical Metrics. J Magn Reson Imaging. 2020 Dec;52(6):1645-1654.
Leutz-Schmidt P, Wielpütz MO, Skornitzke S, Weinheimer O, Kauczor HU, Puderbach MU, Pahn G, Stiller W. Influence of acquisition settings and radiation exposure on CT lung densitometry-An anthropomorphic ex vivo phantom study. PLoS One. 2020 Aug 14;15(8).
Konietzke P, Wielpütz MO, Wagner WL, Wuennemann F, Kauczor HU, Heussel CP, Eichinger M, Eberhardt R, Gompelmann D, Weinheimer O. Quantitative CT detects progression in COPD patients with severe emphysema in a 3-month interval. Eur Radiol. 2020 May;30(5):2502-2512.
Faller FK, Mein S, Ackermann B, Debus J, Stiller W, Mairani A. Pre-clinical evaluation of dual-layer spectral computed tomography-based stopping power prediction for particle therapy planning at the Heidelberg Ion Beam Therapy Center. Phys Med Biol. 2020 Apr 24;65(9):095007.
Jobst BJ, Weinheimer O, Buschulte T, Trauth M, Tremper J, Delorme S, Becker N, Motsch E, Groß ML, Trotter A, Eichinger M, Kauczor HU, Wielpütz MO. Longitudinal airway remodeling in active and past smokers in a lung cancer screening population. Eur Radiol. 2019 Jun;29(6):2968-2980.
Stiller W. Basics of iterative reconstruction methods in computed tomography: A vendor-independent overview. Eur J Radiol. 2018 Dec;109:147-154.
Palm V, Sheng R, Mayer P, Weiss KH, Springfeld C, Mehrabi A, Longerich T, Berger AK, Kauczor HU, Weber TF. Imaging features of fibrolamellar hepatocellular carcinoma in gadoxetic acid-enhanced MRI. Cancer Imaging. 2018 Mar 1;18(1):9.
Pahn G, Skornitzke S, Schlemmer HP, Kauczor HU, Stiller W. Toward standardized quantitative image quality (IQ) assessment in computed tomography (CT): A comprehensive framework for automated and comparative IQ analysis based on ICRU Report 87. Phys Med. 2016 Jan;32(1):104-15.
Stiller W, Skornitzke S, Fritz F, Klauss M, Hansen J, Pahn G, Grenacher L, Kauczor HU. Correlation of quantitative dual-energy computed tomography iodine maps and abdominal computed tomography perfusion measurements: are single-acquisition dual-energy computed tomography iodine maps more than a reduced-dose surrogate of conventional computed tomography perfusion? Invest Radiol. 2015 Oct;50(10):703-8.
Triphan SM, Jobst BJ, Breuer FA, Wielpütz MO, Kauczor HU, Biederer J, Jakob PM. Echo time dependence of observed T1 in the human lung. J Magn Reson Imaging. 2015 Sep;42(3):610-6.
Weinheimer O, Achenbach T, Bletz C, Duber C, Kauczor HU, Heussel CP. About objective 3-d analysis of airway geometry in computerized tomography. IEEE Trans Med Imaging. 2008 Jan;27(1):64-74.