Die AG Ganter im Detail
Aktuelle Mitarbeiter*innen
Dr. Markus Ganter (AG-Leiter, seit 2016)
Marie Jakobovitz, Ph.D. (Postdoc in Kollaboration mit dem EMBL, seit 2023)
Aiste Kudulyte (Doktorandin, seit 2023)
Laura Plutowski-Wrobel (MSc-Arbeit, 2022 - 2023; HiWi, 2023 - 2024; Doktorandin, seit 2024)
Leonie Schön (med. Doktorarbeit, seit 2025)
Muriel Lauer (MSc-Arbeit, seit 2025)
& wechselnde BSc Studierende :-)
Forschungsschwerpunkt
Normalerweise vermehren sich Zellen, indem sie ihr Genom duplizieren und sich in zwei Tochterzellen teilen. Doch bei einigen Organismen erfolgt die Zellvermehrung auf andere, etwas bizarre Weise. Einer dieser eher ungewöhnlichen Wege ist bei Plasmodium spp. zu beobachten, dem Erreger der Malaria, die nach wie vor eine große Geißel der Menschheit ist.
Ein ungewöhnliches Merkmal der Replikation von Plasmodium in Erythrozyten, der sogenannten Schizogonie, ist die Anzahl der Zellkerne und damit der Tochterzellen, die sie während einer Runde der intrazellulären Replikation produzieren. Parasiten mit einer ungeraden Anzahl von Zellkernen sind leicht zu erkennen (Abb. 1). Die Abweichung von einer geometrischen Expansion, die zu 2, 4, 8, 16 oder 32 Tochterzellen führen würde, wird durch asynchrone Replikation verursacht.

Dies deutet darauf hin, dass sich einzelne Kerne autonom replizieren bis ein globaler Mechanismus übernimmt und die Bildung von Tochterzellen koordiniert. Dies steht in auffälligem Gegensatz zur Kernteilung in anderen vielkernigen Zellen, wie dem frühen Drosophila-Embryo, wo die Kerne einer geometrischen Expansion folgen, da ihre Teilung relativ synchron verläuft.
Um einen Einblick in die Regulierung der Replikation von Plasmodium falciparum zu erhalten, führten wir einen vorwärtsgenetischen Screen für die bedingte Proteinexpression von Proteinkinasen durch und identifizierten eine Kinase als wesentlich für die Vermehrung (Abb. 2)
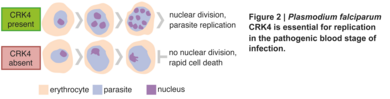
Diese Kinase ist Plasmodium-spezifisch und ein entscheidender Regulator der kontinuierlichen Runden der DNA-Replikation, der Histon-Modifikation sowie der Regulierung der Genexpression. Darüber hinaus konnte nachgewiesen werden, dass diese Kinase für die Übertragung auf die Mücke erforderlich ist. Die Ergebnisse dieser Arbeit dienen als Ausgangspunkt für ein besseres Verständnis der Replikationsbiologie von Plasmodium.
Projekte
Derzeit verwenden wir den menschlichen Erreger Plasmodium falciparum, um die Signalkaskade aufzudecken, die die Vermehrung des Parasiten in der Blutphase der Infektion steuert. Zudem deuten unsere bisherigen Arbeiten darauf hin, dass eine Reihe unbekannter Gene an der Replikation des Parasiten beteiligt ist. Um die Funktion dieser unbekannten Gene zu charakterisieren, wenden wir klassische revers-genetische Methoden und induzierbare Gen-Deletionsansätze an. Des Weiteren erfolgt eine Analyse der räumlich-zeitlichen Organisation der Schizogonie mittels Mikroskopie lebender Zellen.
Das übergeordnete Ziel unserer Forschung ist die Aufklärung der regulatorischen Mechanismen der Plasmodium-Replikation sowie die Erlangung eines Verständnisses für einen Prozess, der mit den derzeitigen Modellen der Zellzyklusregulation scheinbar nicht erklärt werden kann.
Unsere gegenwärtigen Forschungsschwerpunkte sind:
- Analyse der Gene, die an der Regulierung der Replikation und Kernteilung beteiligt sind
- Definition des Interaktom der Plasmodium-spezifischen Kinase CRK4
- Entschlüsselung der raumzeitlichen Organisation der Schizogonie
Publikationen
Preprints
Dey G, Ganter M, Jacobovitz M (2025) Plasticity and scaling through multinucleation: a key adaptation to challenging environments. EcoEvoRxiv, doi.org/10.32942/X2MH1R (currently in revision, Current Biology)
Zink L, Reuschenbach V, Sid Ahmed S, Lauer M, Niethammer J, Kudulyte A, Zorko K, Fackler OT, Hentzschel F, Ganter M (2025) A genetically encoded marker for imaging membranes of Plasmodium. bioRxiv,doi.org/10.1101/2025.10.24.684113 (currently in revision, Molecular Microbiology)
Binder P, Kudulyte A, Klaus S, Höfter T, Schwarz USS, †Ganter M, †Becker N. Competitive resource allocation drives asynchronous and rapid nucelar multiplication in the malaria parasite. bioRxiv, doi.org/10.1101/2025.08.07.669072 (2025) (currently in revision, Nature Communications)
Originalarbeiten & Reviews
2026
Malych R, Bordat Y, Klanicová K, Arbon D, Zahedifard F, Šipková A, Drncová E, Levytska V, Mach J, Plutowski-Wrobel L, Machado M, Štursa J, Truksa J, Ganter M, Sojka D, Zoltner M, Besteiro S, Werner L, Sutak R (2026) Modular Design of Mitochondrion-Targeted Iron Chelators Allows Highly Selective Antiparasitic Activity against Trypanosomes and Apicomplexan Parasites. ACS Infect Dis. Jan 9;12(1):119-127. doi: 10.1021/acsinfecdis.5c00548 Epub 2025 Dec 22. PMID: 41428959; PMCID: PMC12797238.
2025
Nikolova V, Linnemannstöns K, Bendel ME, Machado M, Ganter B, Budimir P, Vogts M, Fischer C, Ganter M, Meier C, Dobbelstein M (2025) Membrane-Permeable 5-Fluorodeoxyuridine Triphosphate Derivatives Inhibit the Proliferation of Plasmodium falciparum. ACS Infect Dis. Nov 14;11(11):3138-3151. doi: 10.1021/acsinfecdis.5c00544 Epub 2025 Oct 3. PMID: 41042506.
Nikolova V, Linnemannstöns K, Zahoruiko A, Ganter M, Lüder CG, Dobbelstein M (2025) Synergistic activity of RSL3 and Pyrimethamine to inhibit the proliferation of Plasmodium falciparum. Antimicrob Agents Chemother. Oct;69(10):e0047125. doi: 10.1128/aac.00471-25 Epub 2025 Aug 18. PMID: 40823867; PMCID: PMC12486841.
Dupouy B, Cotos L, Binder A, Slavikova L, Rottmann M, Mäser P, Jacquemin D, Ganter M, Davioud-Charvet E, Elhabiri M (2025) Click Coupling of Flavylium Dyes with Plasmodione Analogues: Towards New Redox-Sensitive Pro-Fluorophores. Chemistry. Jan 27;31(6):e202403691. doi: 10.1002/chem.202403691 Epub 2024 Dec 16. PMID: 39654502; PMCID: PMC11771622.
2024
Darif ND, Ganter M, Dziekan JM, Kilian N, Brancucci N, Ng C, de Vries LE, Guttery D, Philip N, Boddey JA, Metwally NG, Okumu F, Kooij TWA, Absalon S, Bryant JM (2024) BioMalPar XX: looking back on, and forward from, 20 years of malaria research. Trends Parasitol. Jul 15:S1471-4922(24)00170-3. doi: 10.1016/j.pt.2024.06.012. Epub ahead of print. PMID: 39013661.
2023
Voß Y, Klaus S, Lichti NP, Ganter M, Guizetti J (2023) Malaria parasite centrins can assemble by Ca2+-inducible condensation. PLoS Pathog. Dec 27;19(12):e1011899. doi: 10.1371/journal.ppat.1011899. PMID: 38150475; PMCID: PMC10775985.
Machado M, Klaus S, Klaschka D, Guizetti J, Ganter M (2023) Plasmodium falciparum CRK4 links early mitotic events to the onset of S-phase during schizogony. mBio. Jun 22:e0077923. doi: 10.1128/mbio.00779-23. Epub ahead of print. PMID: 37345936.
Wenz C, Simon CS, Romão TP, Stürmer VS, Machado M, Klages N, Klemmer A, Voß Y, Ganter M, Brochet M, Guizetti J (2023) An Sfi1-like centrin-interacting centriolar plaque protein affects nuclear microtubule homeostasis. PLoS Pathog. May 2;19(5):e1011325. doi: 10.1371/journal.ppat.1011325. PMID: 37130129; PMCID: PMC10180636.
Voß Y, Klaus S, Guizetti J, Ganter M (2023) Plasmodium schizogony, a chronology of the parasite's cell cycle in the blood stage. PLoS Pathog. Mar 2;19(3):e1011157. doi: 10.1371/journal.ppat.1011157. PMID: 36862652; PMCID: PMC9980825. Review
2022
Ganter M†, Guizetti J† and Kilian N† (2022) Visualization of Infected Red Blood Cell Surface Antigens by Fluorescence Microscopy Methods Mol Biol. 2470:425-433. doi: 10.1007/978-1-0716-2189-9_31. PMID: 35881363. †Corresponding authors
Arbon D, Ženíšková K, Šubrtová K, Mach J, Štursa J, Machado M, Zahedifard F, Leštinová T, Hierro-Yap C, Neuzil J, Volf P, Ganter M, Zoltner M, Zíková A, Werner L, Sutak R. (2022) Repurposing of MitoTam: Novel Anti-Cancer Drug Candidate Exhibits Potent Activity against Major Protozoan and Fungal Pathogens. Antimicrob Agents Chemother. Aug 16;66(8):e0072722. doi: 10.1128/aac.00727-22. Epub 2022 Jul 20. PMID: 35856666; PMCID: PMC9380531.
Vandorpe DH, Rivera A, Ganter M, Dankwa S, Wohlgemuth JG, Dlott JS, Snyder LM, Brugnara C, Duraisingh M, Alper SL. (2022) Purinergic signaling is essential for full Psickle activation by hypoxia and by normoxic acid pH in mature human sickle red cells and in vitro-differentiated cultured human sickle reticulocytes. Pflugers Arch. May;474(5):553-565. doi: 10.1007/s00424-022-02665-z. Epub 2022 Feb 16. PMID: 35169901.
Klaus S, Binder P, Kim J, Machado M, Funaya C, Schaaf V, Klaschka D, Kudulyte A, Cyrklaff M, Laketa V, Höfer T, Guizetti J, Becker NB, Frischknecht F, Schwarz US, Ganter M. (2022) Asynchronous nuclear cycles in multinucleated Plasmodium falciparum facilitate rapid proliferation. Sci Adv. Apr;8(13):eabj5362. doi: 10.1126/sciadv.abj5362. Epub 2022 Mar 30. PMID: 35353560.
2021
Schumann R, Bischoff E, Klaus S, Möhring S, Flock J, Keller S, Remans K, Ganter M, Deponte M. (2021) Protein abundance and folding rather than the redox state of Kelch13 determine the artemisinin susceptibility of Plasmodium falciparum. Redox Biol. Oct 31;48:102177. doi: 10.1016/j.redox.2021.102177. Epub ahead of print. PMID: 34773836; PMCID: PMC8600086.
Simon CS, Funaya C, Bauer J, Voβ Y, Machado M, Penning A, Klaschka D, Cyrklaff M, Kim J, Ganter M, Guizetti J. (2021) An extended DNA-free intranuclear compartment organizes centrosome microtubules in malaria parasites. Life Sci Alliance. Sep 17;4(11):e202101199. doi: 10.26508/lsa.202101199. PMID: 34535568; PMCID: PMC8473725.
Ganter M, Frischknecht F. (2021) Illuminating Plasmodium invasion by lattice-light-sheet microscopy. Trends Parasitol. Sep;37(9):777-779. doi: 10.1016/j.pt.2021.07.008. Epub 2021 Jul 28. PMID: 34332896.
Machado M, Steinke S, Ganter M. (2021) Plasmodium Reproduction, Cell Size, and Transcription: How to Cope With Increasing DNA Content? Front Cell Infect Microbiol. Apr 9;11:660679. doi: 10.3389/fcimb.2021.660679. PMID: 33898332; PMCID: PMC8062723.
2020
Pape C, Remme R, Wolny A, Olberg S, Wolf S, Cerrone L, Cortese M, Klaus S, Lucic B, Ullrich S, Anders-Össwein M, Wolf S, Cerikan B, Neufeldt CJ, Ganter M, Schnitzler P, Merle U, Lusic M, Boulant S, Stanifer M, Bartenschlager R, Hamprecht FA, Kreshuk A, Tischer C, Kräusslich HG, Müller B, Laketa V. (2020) Microscopy-based assay for semi-quantitative detection of SARS-CoV-2 specific antibodies in human sera: A semi-quantitative, high throughput, microscopy-based assay expands existing approaches to measure SARS-CoV-2 specific antibody levels in human sera. Bioessays. 2021 Mar;43(3):e2000257. doi: 10.1002/bies.202000257. Epub 2020 Dec 30. PMID: 33377226; PMCID: PMC7883048.
Quadt K, Smyrnakou X, Frischknecht F, Böse G, Ganter M. (2020) Plasmodium falciparum parasites exit the infected erythrocyte after haemolysis with saponin and streptolysin O. Parasitology Research. 119:4297–4302 (doi: 10.1007/s00436-020-06932-9)
Earlier papers
Przyborski J, Ganter M. (2018). Das ungewöhnliche Leben der Malariaparasiten. Biol. Unserer Zeit 48, 162–169 (invited review article in German)
Ganter M, Goldberg JM, Dvorin JD, Paulo JA, King JG, Tripathi AK, Paul AS, Yang J, Coppens I, Jiang RHY, Baker DA, Dinglasan RR, Gygi SP, Duraisingh MT. (2017) Plasmodium falciparum CRK4 directs continuous rounds of DNA replication during schizogony. Nature Microbiology 2: 17017 (DOI: 10.1038/nmicrobiol.2017.17)
Paul AS, Saha S, Jiang RHY, Coleman BI, Kosber AL, Chen C, Ganter M, Espy N, Gubbels MJ, Duraisingh MT. (2015) Parasite calcineurin regulates host cell recognition and attachment by apicomplexans. Cell Host Microbe 18: 49–60 (http://www.cell.com/cell-host-microbe/abstract/S1931-3128(15)00251-6)
Ganter M, Rizopoulos Z, Schuler H, Matuschewski K. (2015) Pivotal and Distinct Role for Plasmodium Actin Capping Protein alpha during Blood Infection of the Malaria Parasite. Molecular Microbiology 96: 84–94 (http://onlinelibrary.wiley.com/doi/10.1111/mmi.12922/full)
Coleman BI, Skillman KM, Jiang RHY, Childs LM, Altenhofen LM, Ganter M, Leung Y, Goldowitz I, Kafsack BFC, Marti M, Llinas M, Buckee CO, Duraisingh MT. (2014) A Plasmodium falciparum Histone Deacetylase Regulates Antigenic Variation and Gametocyte Conversion. Cell Host Microbe 16: 177–186 (http://www.cell.com/cell-host-microbe/abstract/S1931-3128(14)00262-5)
Sattler* J, Ganter* M, Hliscs M, Matuschewski K, Schuler H. (2011) Actin regulation in the malaria parasite. European Journal of Cell Biology 90: 966–971 (http://www.sciencedirect.com/science/article/pii/S017193351000261X) * Equal contribution
Siden-Kiamos* I, Ganter* M, Kunze A, Hliscs M, Steinbüchel M, Mendoza J, Sinden R, Louis K, Matuschewski K. (2011) Stage-specific depletion of myosin A supports an essential role in motility of malarial ookinetes. Cellular Microbiology 13: 1996-2006 (http://onlinelibrary.wiley.com/doi/10.1111/j.1462-5822.2011.01686.x/full) * Equal contribution
Ganter M, Schuler H, Matuschewski K. (2009) Vital role for the Plasmodium capping protein (CP) beta subunit in motility of malaria sporozoites. Molecular Microbiology 74: 1356-1367 (http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2958.2009.06828.x/full)
Kursula I, Kursula P, Ganter M, Panjikar S, Matuschewski K, Schuler H. (2008) Structural Basis for Parasite-Specific Functions of the Divergent Profilin of Plasmodium falciparum. Structure 16: 1638-1648 (http://www.sciencedirect.com/science/article/pii/S0969212608003754)
News - AG Ganter
Markus Ganter im Interview
(25. Februar 2025)
Im Rahmen des gemeinsamen Projekts “Überlebensstrategien” der lebenswissenschaftlichen Forschungsverbünde der Universität Heidelberg mit dem Rhein-Neckar-Fernsehen (RNF) wurden stellvertretend für den Sonderforschungsbereich 1129 zwei AG-Leiter*innen unseres Zentrums interviewt: Frauke Mücksch (Virologie) sowie Markus Ganter aus unserer Abteilung. Ein sehr informatives Gespräch zu Viren und Parasiten, welches wir uneingeschränkt empfehlen - genauso natürlich wie die weiteren Interviews in der Reihe.
Akad. Mittagspause
(6. Mai - 19. Juli 2024)
15-minütige Fachvorträge, fernab des Hörsaals in der besonderen Atmosphäre der Peterskirche gehalten, unterhaltsam, lehrreich und vor allen Dingen leicht verständlich, mit anschließender Frage- und Gesprächsrunde. 2024 gewährte die Health + Life Science Alliance Heidelberg Mannheim Einblicke in die aktuelle Spitzenforschung in den Lebenswissenschaften, die Behandlung menschlicher Krankheiten sowie die wissenschaftlichen Grundlagen für neue Therapien.
Die Veranstaltungen fanden montags bis freitags (außer feiertags) von 13.00 Uhr bis 13.30 Uhr in der Heidelberger Peterskirche, Plöck 70, statt.
Achtung: am 7. Juni hat unser AG-Leiter Markus Ganter einen Vortrag zum Thema “Die ungewöhnliche Lebensweise des Malariaparasiten” gehalten, welcher ebenfalls online auf YouTube zur Verfügung steht.




