Radiopharmazeutische Chemie
Prinzipien der Radiopharmazie
Therapies based on radionuclides
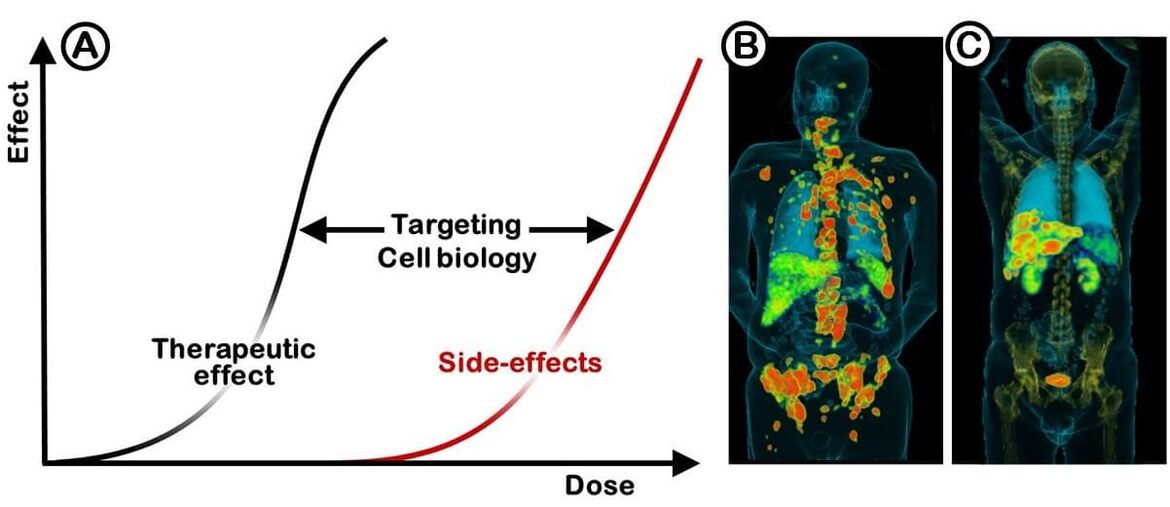
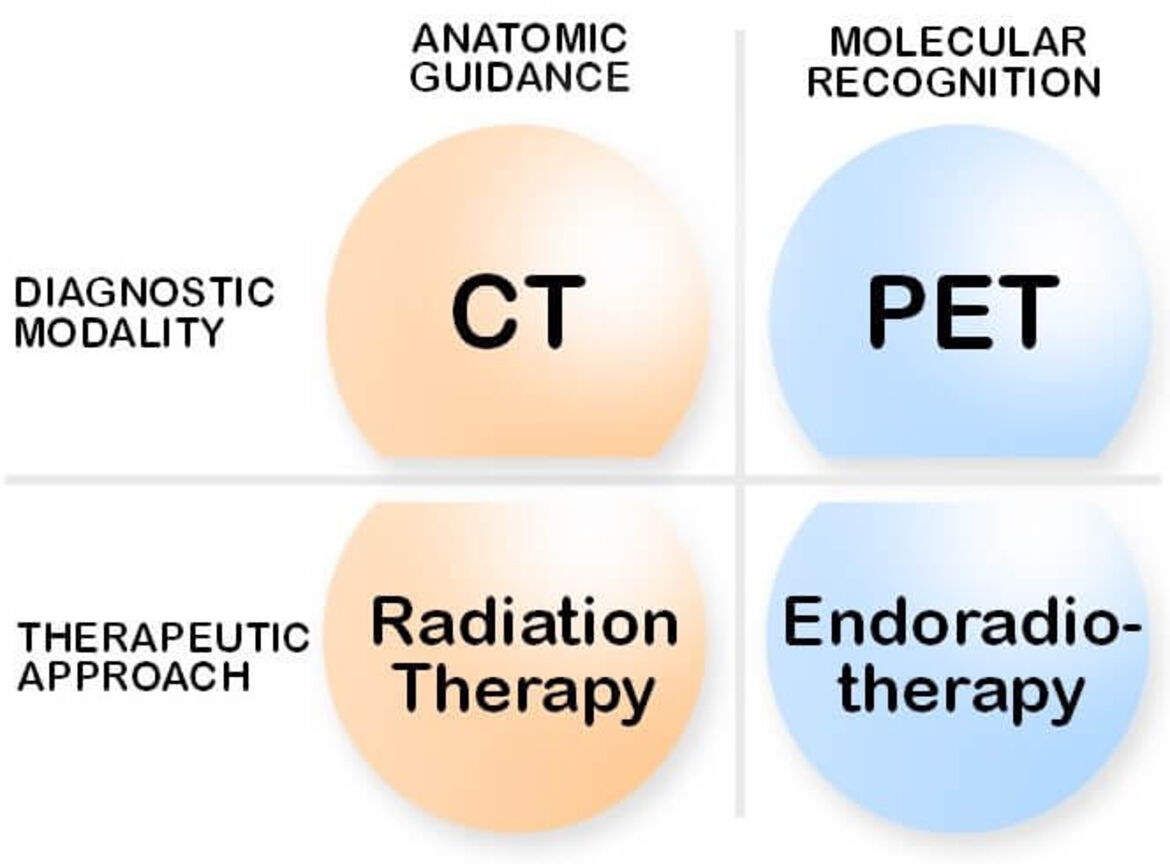
Molecular imaging plays a pivotal role in todays individualized therapy strategies. Beyond this, the tracers used in nuclear medicine procedures provide the possibility to achieve tumor selective therapeutics. The efficiency of systemic tumor treatments is limited by the side effects. As a result, the value of any treatment modality is defined by specificity of the effect on the tumor versus the dose limiting organs. The ratios achieved with the standard chemotherapeutics are not sufficient for an effective treatment of most solid tumors. Radionuclides can be used for treatment purposes in analogy to radiation therapies combined with radiological imaging methods such as CT (Figure 1).
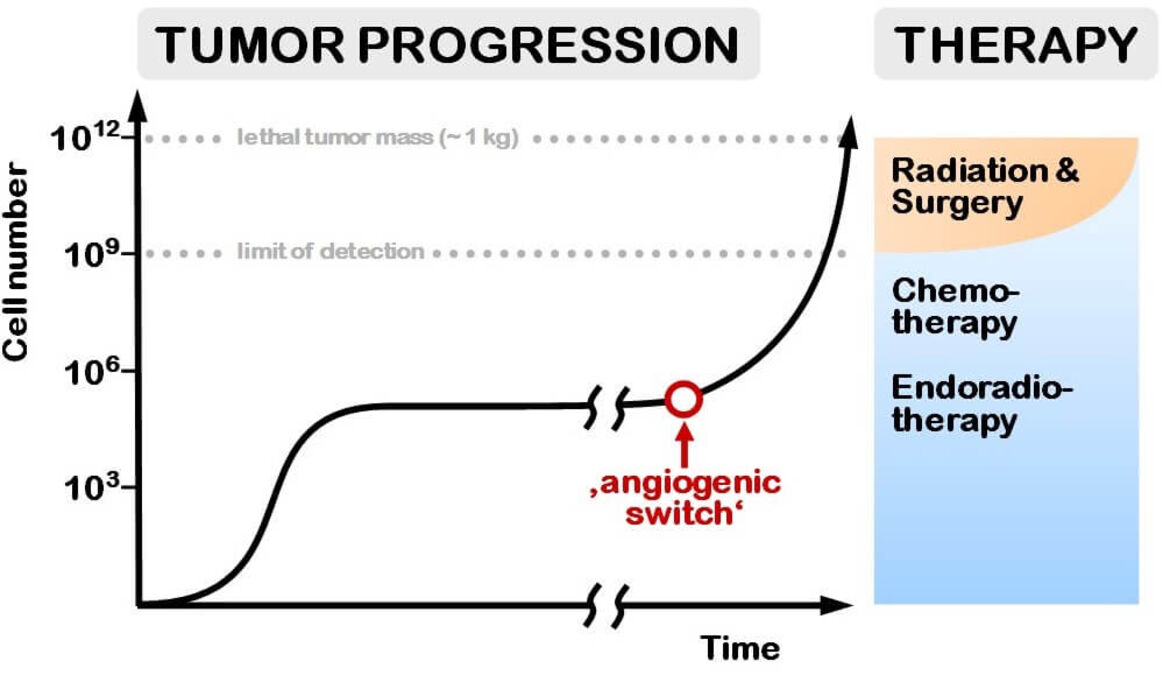
This can be exploited to improve the limits of the current treatment of solid tumors. As shown in Figure 2, surgery and radiation therapy, still the main treatment modalities can only be applied to tumors above the limit of detection (typically 1 cm in diameter). These tumors are generally a very aggressive phenotype, disseminated disease is not amenable to both modalities. Once metastasized, the treatment of choice is drug therapy. However, the standard chemotherapeutics do not achieve selective tumor uptake.
The application of targeted therapies using cytotoxic radioisotopes (alpha or beta emitting isotopes) has been shown to provide the ratios required to treat patients with different tumors that were resistant to all other therapies and to demonstrate the possibility to go beyond the potential of all known systemic treatment modalities. Prime examples for this efficacy are 90Y-DOTATOC (neuroendocrine tumors), 90Y-Zevalin (non-Hodgkin lymphoma), 131I-Benzamides (melanoma), 131I-Iodide (cancers of the thyroid), 223Ra-Xofigo (prostate tumor metastases) and 131I-MIP1466 (prostate tumors). As visualized in Figure 3 this presents a groundbreaking option for the therapy of oncological diseases. Generally, two strategies can be followed to enhance the efficacy of tumor therapies. As the efficacy of any tumor treatment is defined by the quotient of the effect on tumor versus the dose limiting organs, these strategies are a) specific action of the cytotoxic agent on tumor cells and b) specific uptake of the cytotoxic agent.