Die MCTU im Detail
Aktuelle Mitarbeiter*innen
Richard Thomson Luque (wissenschaftlicher Leiter, seit 2021)
Kristin Fürle (leitende technische Assistentin, seit 2021)
Viktoria Kiehl (technische Assistentin, seit 2023)
Inken Rasmussen (med. Doktorarbeit, 2024)
& wechselnde BSc Studierende
Alumni
Bruno Vellutini (MSc Praktikum, 2024)
Alejandro Vanazzi (MSc Praktikum, 2024)
Lara Veitl (BSc Praktikum, 2024)
Catherine Mkindi (Gastwissenschaftlerin, 2024)
Veronika Rathay (med. Doktorarbeit, 2023 - 2024)
Madeleine Bühlmeyer (BSc Arbeit, 2023)
Lea Woltereck (MSc Praktikum, 2023)
Anne Ulmer (med. Doktorarbeit, 2022 - 2023)
Arin Ali (Qualitätsmanagement, 2022 - 2023)
Maximilian Winter (BSc Arbeit, 2022)
Julia Hibbert (Doktorandin, 2022)
Micha Rosenkranz (Doktorand bei Prof. Dr. Faith Osier, 2021 - 2022)
Kalina Chavdarova (BSc Arbeit, 2021)
Natascha Osswald (BSc Arbeit, 2021)

Forschungsschwerpunkt
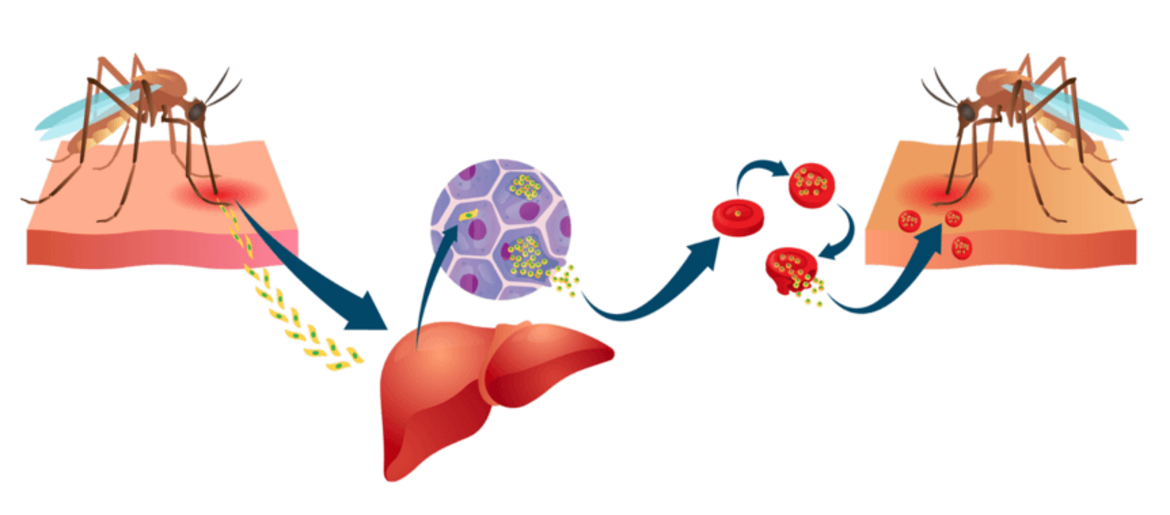
Malaria wird durch Parasiten verursacht, die durch Mückenstiche übertragen werden. Das erste Ziel des Parasiten nach dem infektiösen Stich ist die Leber. Die massive Vermehrung des Parasiten in der Leber und seine Freisetzung in den Blutkreislauf führt zum Blutstadium der Infektion, wo die Parasiten die roten Blutkörperchen infizieren. Dort vermehren sie sich erneut, töten die Wirtszellen ab und infizieren neue rote Blutkörperchen. Dieser Zyklus des Blutstadiums verursacht alle pathophysiologischen Symptome und führt bei ungeschützten, "naiven" Personen häufig zum Tod. Die MCTU arbeitet Seite an Seite mit der Sumaya-Biotech GmbH & Co. KG an der Entwicklung wirksamer Malaria-Impfstoffe und ergänzender Medikamente, die für das Ziel der Ausrottung der Malaria unerlässlich erscheinen.
Projekte
Die Malaria-Impfstoffkandidaten von Sumaya Biotech basieren auf der vollen Länge des Merozoiten-Oberflächenproteins 1 (MSP1), welches an der Invasion der roten Blutkörperchen durch den Parasiten und an der Freisetzung von Tochterzellen aus den infizierten Erythrozyten beteiligt ist. MSP1 wird auch während des Leberstadiums des Parasiten exprimiert.
Sum-101
(vormals SumayaVac1) basiert auf dem rekombinanten 196kDa MSP1-Protein zusammen mit einem Adjuvans (GLA-SE) und wurde in einer ersten Studie in Heidelberg erfolgreich am Menschen igetestet. Der Impfstoff erwies sich als sicher, gut verträglich und immunogen (=fähig, eine Immunantwort auszulösen), wobei alle Geimpften Antikörper bildeten.
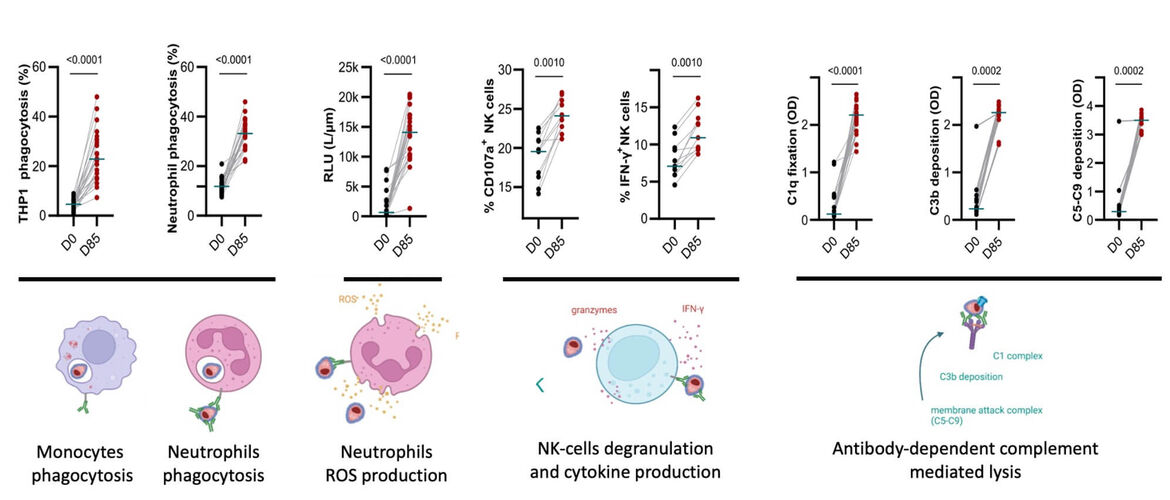
Die von SUM-101 ausgelösten Antikörper waren in der Lage, verschiedene Fc-vermittelte Effektormechanismen zu stimulieren, die mit dem Schutz vor Malaria in Zusammenhang stehen, darunter Phagozytose, Freisetzung reaktiver Sauerstoffspezies, Produktion von IFN-γ sowie Komplementaktivierung und -fixierung. Die multifunktionale Aktivität der humoralen Immunantwort blieb mindestens sechs Monate nach der Impfung erhalten und war vergleichbar mit der von natürlich erworbenen Anti-MSP1-Antikörpern bei halbimmunen Erwachsenen aus Kenia. Eine zweite klinische Studie, die in Tansania an vorexponierten Freiwilligen durchgeführt wurde, ist derzeit im Gange.
SUM-102
ist ein Adenovirus, das MSP-1 exprimiert und zusammen mit SUM-101 in einem Prime-Boost-Schema eingesetzt werden soll. Es wurde bereits mit regulatorischen toxikologischen Studien begonnen.
Aktivitäten
BioMalPar (Mai 2023)
Auf der XIV. BioMalPar Konferenz, die vom 23. bis 25. Mai 2023 am EMBL in Heidelberg stattfand, präsentierte die MCTU ein Poster zum Thema "Immunisierung mit dem Plasmodium falciparum MSP1 Malaria-Impfstoffkandidaten SumayaVac-1 löst multifunktionale IgMs aus”.
BNITM Malaria Meeting (März 2023)
Das Malaria Meeting am Bernhard-Nocht-Institut für Tropenmedizin (BNITM) hat sich im Laufe der Jahre zu einer über die Grenzen Deutschlands hinaus beachteten wissenschaftlichen Veranstaltung entwickelt, bei der sowohl Kliniker als auch nicht-klinische Naturwissenschaftler ihre Ergebnisse zur aktuellen Malariaforschung vorstellen, unter anderem 2023 die MCTU.

ICTM (Oktober 2022)

Im Oktober 2022 stellte die MCTU die neuesten Ergebnisse zu SUM-101 (SumayaVac1) auf dem 20. Internationalen Kongress für Tropenmedizin und Malaria (ICTMM) in Bangkok vor. Der Impfstoff löste FC-vermittelte Effektor-Funktionen sowohl durch IgG als auch durch IgM aus. Es wurden Ergebnisse zum hohen Grad der Multifunktionalität von Antikörpern gegen die hochkonservierte N-terminale p83-Untereinheit diskutiert. Insbesondere präsentierte die MCTU die Fähigkeit von SUM-101, eine Gedächtnis-T-Zell-Antwort auszulösen und zelluläre Zytotoxizität durch CD8+ T-Zellen zu induzieren.
BioMalPar (Mai 2022)

Vom 23. bis 25. Mai 2022 nahm die MCTU an der XVIII. BioMalPar-Konferenz zur Biologie und Pathologie des Malariaparasiten am EMBL in Heidelberg teil. Richard Thomson Luque präsentierte die Ergebnisse der ersten klinischen Studie SUM-101 (SumavaVac1) am Menschen und stellte insbesondere die neuesten Ergebnisse vor, die zeigen, dass IgG und IgM der Geimpften in der Lage sind, ein breites Spektrum an lang anhaltenden FC-vermittelten Effektor-Funktionen zu entfalten, die mit der natürlichen Immunität der endemisch exponierten Bevölkerung vergleichbar sind.
Publikationsliste
Artikel mit Bezug auf die MCTU
Rathay V, Fürle K, Kiehl V, Ulmer A, Lanzer M, Thomson-Luque R. IgG Subclass Switch in Volunteers Repeatedly Immunized with the Full-Length Plasmodium falciparum Merozoite Surface Protein 1 (MSP1). Vaccines. 2024; 12(2):208. doi.org/10.3390/vaccines12020208
Thomson-Luque R, Stabler TC, Fürle K, Silva JC, Daubenberger C. Plasmodium falciparum merozoite surface protein 1 as asexual blood stage malaria vaccine candidate. Expert Rev Vaccines. 2024 Jan-Dec;23(1):160-173. doi: 10.1080/14760584.2023.2295430. Epub 2023 Dec 27. PMID: 38100310.
Rosenkranz M, Fürle K, Hibbert J, Ulmer A, Ali A, Giese T, Blank A, Haefeli WE, Böhnlein E, Lanzer M, Thomson-Luque R. Multifunctional IgG/IgM antibodies and cellular cytotoxicity are elicited by the full-length MSP1 SumayaVac-1 malaria vaccine. NPJ Vaccines. 2023 Aug 9;8(1):112. doi: 10.1038/s41541-023-00701-2. PMID: 37558673; PMCID: PMC10412566.
Blank, A., et al. Immunization with full-length Plasmodium falciparum merozoite surface protein 1 is safe and elicits functional cytophilic antibodies in a randomized first-in-human trial. NPJ Vaccines 5, 10 (2020).
Frühere Artikel
2021
Thomson-Luque R, Votborg-Novél L, Ndovie W, Andrade CM, Niangaly M, Attipa C, Lima NF, Coulibaly D, Doumtabe D, Guindo B, Tangara B, Maiga F, Kone AK, Traore K, Kayentao K, Ongoiba A, Doumbo S, Thera MA, Traoré B, Seydel K, Osório NS, Portugal S. Plasmodium falciparum transcription in different clinical presentations of malaria associates with circulation time of infected erythrocytes. Nat Commun. 2021 Jul 30;12(1):4711. doi: 10.1038/s41467-021-25062-z. PMID: 34330920; PMCID: PMC8324851.
Thomson-Luque R, Bautista JM. Home Sweet Home: Plasmodium vivax-Infected Reticulocytes-The Younger the Better? Front Cell Infect Microbiol. 2021 May 13;11:675156. doi: 10.3389/fcimb.2021.675156. PMID: 34055670; PMCID: PMC8162270
2020
Andrade CM, Fleckenstein H, Thomson-Luque R, Doumbo S, Lima NF, Anderson C, Hibbert J, Hopp CS, Tran TM, Li S, Niangaly M, Cisse H, Doumtabe D, Skinner J, Sturdevant D, Ricklefs S, Virtaneva K, Asghar M, Homann MV, Turner L, Martins J, Allman EL, N'Dri ME, Winkler V, Llinás M, Lavazec C, Martens C, Färnert A, Kayentao K, Ongoiba A, Lavstsen T, Osório NS, Otto TD, Recker M, Traore B, Crompton PD, Portugal S. Increased circulation time of Plasmodium falciparum underlies persistent asymptomatic infection in the dry season. Nat Med. 2020 Dec;26(12):1929-1940. doi: 10.1038/s41591-020-1084-0. Epub 2020 Oct 26. PMID: 33106664
2019
Adapa SR, Taylor RA, Wang C, Thomson-Luque R, Johnson LR, Jiang RHY. Plasmodium vivax readiness to transmit: implication for malaria eradication. BMC Syst Biol. 2019;13(1):5. Published 2019 Jan 11. doi:10.1186/s12918-018-0669-4 Thomson-Luque R, Adams JH, Kocken CHM, Pasini EM. From marginal to essential: the golden thread between nutrient sensing, medium composition and Plasmodium vivax maturation in in vitro culture. Malar J. 2019;18(1):344. Published 2019 Oct 10. doi:10.1186/s12936-019-2949-x
2018
Richard Thomson-Luque, Chengqi Wang, Francis B. Ntumngia, Shulin X, Karoly Szekeres, Amy Conway, Swamy Rakesh Adapa, Samantha J. Barnes, John H. Adams and Rays H.Y. Jiang. In depth phenotypic characterization of reticulocyte maturation using mass cytometry. Blood Cells Molecules and Diseases July 2018. DOI: 10.1016/j.bcmd.2018.06.004
Alison Roth, Steven P. Maher, Amy J. Conway, Ratawan Ubalee, Victor Chaumeau, Chiara Andolina, Stephen A. Kaba, Amélie Vantaux, Malina A. Bakowski, Richard Thomson-Luque, Swamy Rakesh Adapa, Naresh Singh, Samantha J. Barnes, Caitlin A. Cooper, Mélanie Rouillier, Case W. McNamara, Sebastian A. Mikolajczak, Noah Sather, Benoît Witkowski, Brice Campo, Stefan H. I. Kappe, David E. Lanar, François Nosten, Silas Davidson, Rays H. Y. Jiang, Dennis E. Kyle, John H. Adams. A comprehensive model for assessment of liver stage therapies targeting Plasmodium vivax and Plasmodium falciparum. Nature Communications. 2018;9:1837. doi:10.1038/s41467-018-04221-9
Ntumngia FB, Thomson-Luque R, Galusic S, Frato G, Frischmann S, Peabody DS, Chackerian B, Ferreira MU, King CL, Adams JH. Identification and immunological charaterization of the ligand domain of Plasmodium vivax reticulocyte binding protein 1a. J Infect Dis. 2018 May 7
2017
Francis B. Ntumngia, Camilla V. Pires, Samantha J. Barnes, Miriam T. George, Richard Thomson-Luque, Flora S. Kano, Jessica R. S. Alves, Darya Urusova, Dhelio B. Pereira, Niraj H. Tolia, Christopher L. King, Luzia H. Carvalho, John H. Adams. An engineered vaccine of the Plasmodium vivax Duffy binding protein enhances induction of broadly neutralizing antibodies. Scientific Reports. 2017;7:13779. doi:10.1038/s41598-017-13891-2
Thomson-Luque R, Saliba, K. S., Kocken, C. H., & Pasini, E. M. “A Continuous, Long-Term Plasmodium Vivax In Vitro Blood-Stage Culture: What Are We Missing?” Trends in Parasitology, 2017, doi:10.1016/j.pt.2017.07.001
2016
Ntumngia FB, Thomson-Luque R, Torres L de M, Gunalan K, Carvalho LH, Adams JH. A Novel Erythrocyte Binding Protein of Plasmodium vivax Suggests an Alternate Invasion Pathway into Duffy-Positive Reticulocytes. mBio. 2016;7(4):e01261-16. doi:10.1128/mBio.01261-16
Shaw-Saliba K*, Thomson-Luque R*, Obaldía N, et al. Insights into an Optimization of Plasmodium vivax Sal-1 In Vitro Culture: The Aotus Primate Model. Sinnis P, ed. PLoS Neglected Tropical Diseases. 2016;10(7):e0004870. doi:10.1371/journal.pntd.0004870. *equally contributed.
2015
Leandro-Reguillo P, Thomson-Luque R, Monteiro WM, de Lacerda MV. Urban and architectural risk factors for malaria in indigenous Amazonian settlements in Brazil: a typological analysis. Malar J. 2015;14:284. Published 2015 Jul 22. doi:10.1186/s12936- 015-0806-0
Thomson-Luque R, Scopel KK. Immature reticulocytes as preferential host cells and the challenges for in vitro culture of Plasmodium vivax. Pathog Glob Health. 2015;109(3):91-92. doi:10.1179/2047772415Z.00000000026
2014
Stefanie C. P. Lopes, Letusa Albrecht, Bruna O. Carvalho, André M. Siqueira, Richard Thomson-Luque, Paulo A. Nogueira, Carmen Fernandez-Becerra, Hernando A. del Portillo, Bruce M. Russell, Laurent Rénia, Marcus V. G. Lacerda, Fabio T. M. Costa, Paucity of Plasmodium vivax Mature Schizonts in Peripheral Blood Is Associated With Their Increased Cytoadhesive Potential, The Journal of Infectious Diseases, Volume 209, Issue 9, 1 May 2014, Pages 1403–1407, https://doi.org/10.1093/infdis/jiu018
2013
Martin-Jaular L, Elizalde-Torrent A, Thomson-Luque R, Ferrer M, Segovia JC, Esperanza Herreros-Aviles, Fernández-Becerra C and del Portillo HA. Reticulocyte prone malaria parasites predominantly invade CD71hi immature cells: implications for the development of an in vitro culture for Plasmodium vivax. Malar J. 2013;12(1):434. doi:10.1186/1475-2875-12-434
Fernandez-Becerra C, Lelievre J, Ferrer M, Anton N, Thomson-Luque R, Peligero C, Almela MJ, Lacerda MV, Herreros E, del Portillo, H. A. (2013). Red blood cells derived from peripheral blood and bone marrow CD34+ human haematopoietic stem cells are permissive to Plasmodium parasites infection. Memórias Do Instituto Oswaldo Cruz, 108(6), 801–803. http://doi.org/10.1590/0074-0276108062013019
2008
Cristian Morales Indiano, Maria-Luisa Granada, Carme Biosca Adzet, Emma Ventura Orriols, Richard Thomson-Luque, August Corominas Vilardell. Estudio de la estabilidad de insulina, testosterona y péptido C en suero y de paratirina en suero y plasma. Revista del Laboratorio Clínico 1(1) SEQC (2008)
News - MCTU
SUM-101 Phase Ib klinische Studie in Bagamoyo, Tansania

Dies ist eine klinische Studie für einen neuen Malaria-Impfstoff, SumayaVac-1 (SUM-101), eine Zusammenarbeit zwischen Sumaya Biotech GmbH & Co. KG, dem Swiss TPH und dem Ifakara Health Institute. An der Studie nehmen gesunde erwachsene afrikanische Freiwillige teil, die in Bagamoyo, Tansania, einer Malaria-Exposition ausgesetzt wurden. Ziel der Studie ist es, die Sicherheit des Impfstoffs und seine Fähigkeit, eine Immunreaktion gegen den Malariaparasiten hervorzurufen, zu bewerten.
Vierzig Teilnehmer, die in zwei Gruppen aufgeteilt werden, erhalten im Abstand von etwa einem Monat drei Dosen entweder des Impfstoffs SUM-101 oder eines Vergleichsimpfstoffs (Tollwutimpfstoff). Der SUM-101-Impfstoff besteht aus dem Merozoiten-Oberflächenprotein (MSP-1), welches auf Merozoiten und im Leberstadium exprimiert wird, und einem starken immunstimulierenden Adjuvans namens GLA-SE (Glucopyranosyl Lipid A Adjuvant-Stable Emulsion). Der Impfstoff zielt gleichzeitig auf die beiden entscheidenden Stadien des Infektionszyklus des Parasiten, das Leber- und das Blutstadium.
EU Malaria Fonds unterstützt Sumaya Biotech
Der EU-Malaria-Fonds (EUMF) ist eine öffentlich-private Partnerschaft zwischen der Europäischen Union, internationalen Organisationen, Unternehmen und der organisierten Zivilgesellschaft. Er bietet ein neuartiges Finanzierungsinstrument zur Behebung von Marktversagen bei Infektionskrankheiten, die für die öffentliche Gesundheit weltweit von großer Bedeutung sind. Am 30. September 2021 schloss der EUMF seine Investitionen in vier innovative Unternehmen ab, die in der Malariaforschung und -entwicklung tätig sind, darunter Sumaya Biotech. Insgesamt wurden neun vielversprechende und innovative Malariaprojekte unterstützt, die Behandlungen, Impfstoffe und Diagnostika umfassen.
Der Schwerpunkt der Sumaya Biotech GmbH & Co. KG liegt auf der klinischen Entwicklung eines neuartigen, innovativen Malaria-Impfstoffs auf der Basis des MSP-1-Proteins. Das zweite Projekt von Sumaya zielt auf die Entwicklung von SC83288, einem Anti-Malaria-Wirkstoff. Die beiden Projekte werden in enger Zusammenarbeit mit unserer Abteilung durchgeführt.