Research Group „Molecular Immunology of Bone Infections“
Group leader Prof. Katharina Kubatzky
Follow us on Twitter @kkubatzky

Back (from left to right): Vincent Ebert, Lienhard Leibolt, Elisabeth Seebach, Stella Langenbach. Front: Sevinj Sultanli, Gabriele Sonnenmoser, Dayoung Yu, Katharina Kubatzky
apl. Prof. Dr. Katharina Kubatzky
Dept. of Medical Microbiology and Hygiene
Im Neuenheimer Feld 324
69120 Heidelberg Germany
Phone +49 6221 56 38361
Fax +49 6221 56 33794
kubatzky(at)uni-heidelberg.de
Mitglied von Infect-Net, dem Verband deutscher Infektionsforscherinnen
https://www.infectnet.org/web/mitglied.php?id=130
Our research interest
We are interested in mechanisms of bacterial immune evasion in the context of bone infections as well as the inflammatory responses mediated by the innate immune system.
Students are welcome for practical courses, Bachelor or Master thesis projects!
Projects
Pasteurella multocida toxin (PMT)
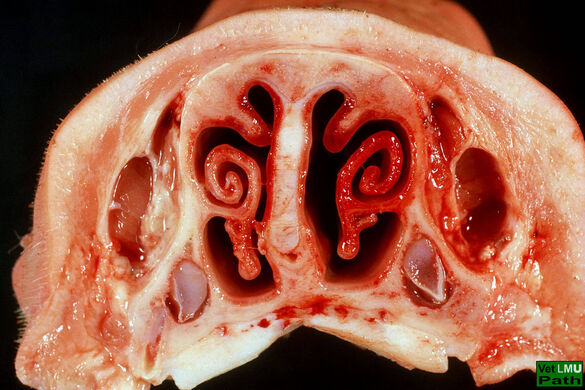
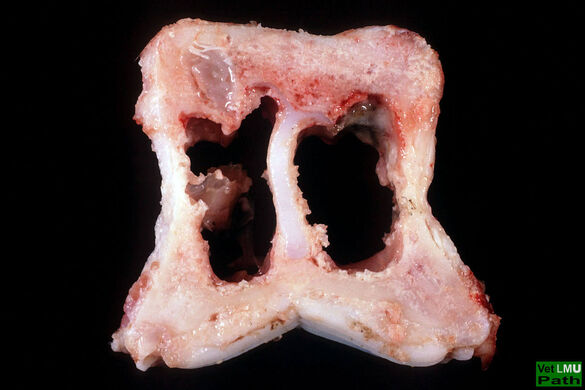
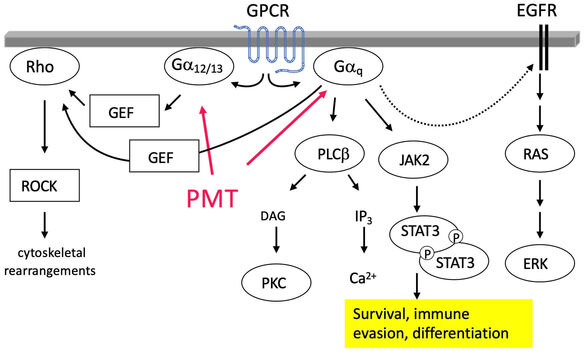
Pasteurella multocida Toxin (PMT) is a protein toxin that causes atrophic rhinitis in pigs, a disease characterized by bone resorption and loss of nasal turbinate bones. PMT is taken up by host cells through receptor-mediated endocytosis and intracellularly constitutively activates heterotrimeric G proteins such as Gq and G12/13 through deamidation. Ultimately this activates signalling cascades that lead to the blocking of bone building osteoblasts and an increased differentiation of bone resorbing osteoclasts.
Osteoclasts are haematopoietic cells that develop under the influence of the cytokines M-CSF and RANKL from the monocyte/macrophage lineage. Under inflammatory conditions, macrophage activity and osteoclast differentiation mutually influence each other, for example through the release of pro-inflammatory cytokines such as IL-6, TNF-alpha and IL-1beta that can also act as osteoclastogenic factors. Recently, the influence of metabolism on macrophage function and plasticity came into focus, however, the impact of metabolism on osteoclast differentiation is less well studied. As bone resorption is an energy demanding process, metabolic activity is a central point in osteoclast differentiation and often found to be deregulated in diseases with increased osteoclast formation
Our previous research found that PMT induces the activation of STAT (signal transducer and activator of transcription) and Pim kinases and that these proteins are key signalling effectors that drive the formation of highly active osteoclasts. Interestingly, STAT3 and Pim proteins are also associated with diseases such as multiple myeloma or arthritis where they take part in the formation of hypermetabolic macrophages. Cell metabolism is already a well-established target in cancer therapy, but has only recently be discovered as an equally interesting target in inflammatory diseases such as rheumatoid arthritis, periodontitis or osteomyelitis. We will investigate the importance of the above-mentioned pathways for osteoclast differentiation and activity, to better understand the intricate interplay between metabolic activity and function.
Modell systems and Techniques:
Primary murine osteoclasts, metabolic assays, western blots, RT-PCR, biochemical cell fractionation, FACS
Selected publications:
- Chakraborty S, Handrick B, Yu D, Bode KA, Hafner A, Schenz J, Schaack D, Uhle F, Tachibana T, Kamitani S, Vogl T, Kubatzky KF
Gαq modulates the energy metabolism of osteoclasts.
Front Cell Infect Microbiol. 2023 Jan 9;12:1016299 - Kubatzky KF
Pasteurella multocida toxin - lessons learned from a mitogenic toxin.
Front Immunol. 2022 Dec 16;13:1058905 - Chakraborty S, Kloos B, Roetz N, Schmidt S, Eigenbrod T, Kamitani S, Kubatzky KF. 2018
Influence of Pasteurella multocida Toxin on the differentiation of dendritic cells into osteoclasts.
Immunobiology. 2018 Jan;223(1):142-150. - Chakraborty S, Kloos B, Harre U, Schett G, Kubatzky KF. 2017
Pasteurella multocida Toxin Triggers RANKL-Independent Osteoclastogenesis.
Front Immunol. 2017 Feb 27;8:185. - Kloos B, Chakraborty S, Lindner SG, Noack K, Harre U, Schett G, Krämer OH, Kubatzky KF. 2015
Pasteurella multocida toxin- induced osteoclastogenesis requires mTOR activation.
Cell Commun Signal. 13:40 (2015). - Hildebrand D, Bode KA, Rieß D, Cerny D, Waldhuber A, Römmler F, Strack F, Korten S, Orth JH, Miethke T, Heeg K, Kubatzky KF. 2014
Granzyme A Produces Bioactive IL-1ß through a Nonapoptotic Inflammasome-Independent Pathway.
Cell Rep. 9(3): 910-917 - Orth JH, Aktories K, Kubatzky KF. 2007
Modulation of host cell gene expression through activation of STAT transcription factors by Pasteurella multocida toxin.
J Biol Chem. 282(5): 3050-3057
Members:
Dayoung Yu (PhD student)
Stella Langenbach (Master student, Uni Essen-Duisburg)
Dr. Bianca Kloos (Alumni)
Dr. Sushmita Chakraborty (Alumni, WTD India, AII New Delhi)
Funding:
SPP1648 Immunobone
Staphylococci in bone infections
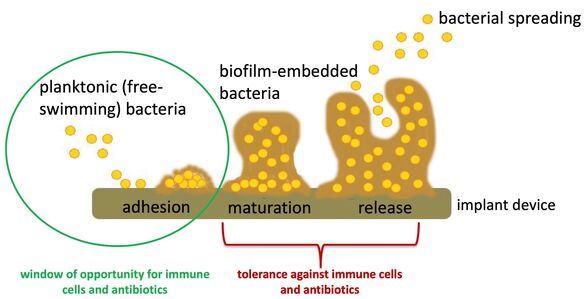
Implant-related bone infections are a feared complication in orthopaedic and trauma surgery. Antibiotic treatment is limited and novel therapeutic strategies are needed. The investigation of endogenous defence strategies is an up-coming research field and modulation of the host immune response is a promising therapeutic approach. As the implant itself impairs the host immune response and bacteria (primarily staphylococci) can take advantage of the implant structure for immune evasion, the analysis of the interactions between implant, immune cell and bacteria, are of great interest. In particular, biofilm formation by bacteria on the implant surface is a major cause for bacterial persistence and chronicity of such infections (see Figure 1).
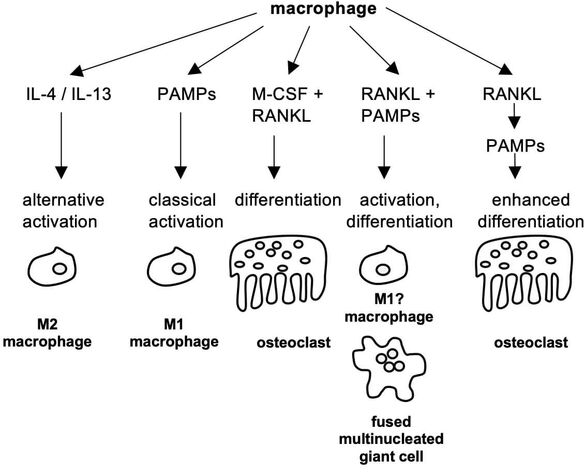
Next to simply shielding the embedded bacteria from immune cell recognition, biofilms are discussed to skew the immune system towards a more tolerant immune response. Our research group is interested in the macrophage function during biofilm formation. Therefore, we culture macrophages within planktonic (free-swimming bacteria) and biofilm environments and investigate their effects on macrophage activity and differentiation. Furthermore, we want to analyse the mechanisms behind the different macrophage phenotypes (see Figure 2).
Our results will contribute to a better understanding of the immune response, which is ineffective to clear bacteria at the planktonic stage and by this unable to prevent biofilm formation and chronicity of implant-related bone infections.
Modell systems and Techniques:
In vitro biofilm formation, preparation of conditioned bacterial media, primary murine macrophages and macrophage cell lines, in vitro formation of macrophage-derived multinucleated giant cells (i.a. osteoclasts), qPCR, FACS, ELISA, Western blots, (bone infection models, µCT)
Selected Publications:
- Seebach E, Elschner T, Kraus FV, Souto-Carneiro M, Kubatzky KF
Bacterial and Metabolic Factors of Staphylococcal Planktonic and Biofilm Environments Differentially Regulate Macrophage Immune Activation.
Inflammation. 2023 Aug;46(4):1512-1530. - Seebach E, Kraus FV, Elschner T, Kubatzky KF
Staphylococci planktonic and biofilm environments differentially affect osteoclast formation.
Inflamm Res. 2023 Jul;72(7):1465-1484. - Seebach E, Sonnenmoser G, Kubatzky KF
Staphylococcus aureus planktonic but not biofilm environment induces an IFN-β macrophage immune response via the STING/IRF3 pathway.
Virulence. 2023 Dec;14(1):2254599. - Seebach E, Kubatzky KF. 2019
Chronic Implant-Related Bone Infections-Can Immune Modulation be a Therapeutic Strategy?
Front Immunol.10:1724. - Kubatzky KF, Uhle F, Eigenbrod T. 2018
From macrophage to osteoclast: How metabolism determines function and activity
Cytokine. 112:102-115. - Seebach E, Holschbach J, Buchta N, Bitsch RG, Kleinschmidt K, Richter W. 2015
Mesenchymal Stromal Cell Implantation for Stimulation of Long Bone Healing Aggravates Staphylococcus Aureus Induced Osteomyelitis
Acta Biomater. 21, 165-77
Members:
Dr. Elisabeth Seebach
Paulina Schad (Alumni)
Katharina Draxel (Alumni)
Funding:
Medizinische Fakultät Heidelberg
Plant-derived molecules with osteoclastogenic properties
Plumbagin is a plant-derived naphthoquinone compound, which has been widely used in eastern traditional medicine. It has gained recent attention in cancer research because of its ability to generate reactive oxygen species and to induce apoptosis of cancer cells. Certain cancer types such as multiple myeloma, breast cancer or prostate cancer frequently metastasise to the bone system and disturb the bone modelling process. Pro-inflammatory cytokines released by the cancer cells or tumour-associated macrophages increase osteoclast activity. Eventually this causes the formation of osteolytic lesions which are a major cause of morbidity and mortality, for example in multiple myeloma patients. Ideally, new therapeutic strategies would therefore target both, tumour as well as bone cells, to prevent osteolysis. The effect of Plumbagin has been investigated in several cancer models, however the studies do not show consistent results on the effects of Plumbagin on osteoclast differentiation and bone density. To better understand the osteoclastic properties of Plumbagin we investigate its function on gene activation and protein levels and the signalling pathways involved.
Modell systems and Techniques:
Primary murine osteoclasts, retroviral transduction, metabolic assays, western blots, RT-PCR, FACS, pulse-chase experiments
Selected publications:
Sultanli S, Schneider J, Burkart SS, Binder M, Kubatzky KF
Cellular ROS tolerance determines the effect of plumbagin on osteoclast differentiation.
FASEB J. 2023 Dec;37(12):e23293.
Yadav AM, Bagade MM, Ghumnani S, Raman S, Saha B, Kubatzky KF, Ashma R
The phytochemical plumbagin reciprocally modulates osteoblasts and osteoclasts.
Biol Chem. 2021 Dec 10;403(2):211-229.
Sultanli S, Ghumnani S, Ashma R, Kubatzky KF.
Plumbagin, a Biomolecule with (Anti)Osteoclastic Properties.
Int J Mol Sci. 2021 Mar 9;22(5):2779. doi: 10.3390/ijms22052779.PMID: 33803472
Members:
Sevinj Sultanli (PhD student)
Funding:
DAAD
Immunometabolism in bacterial infections
In mammalian cells, bone cells regulate the skeleton while the immune system deals with the detection and destruction of invading pathogens. Interestingly, there is a strong crosstalk between these two systems that led scientists to define the emerging field of osteoimmunology. During inflammatory conditions, especially when chronic, bone loss can be observed due to increased osteoclast formation and activity. Macrophage function is influenced by their metabolic activity. While pro-inflammatory M1 macrophages obtain their energy through glycolysis and fatty acid oxidation, anti-inflammatory M2 macrophages rely on oxidative phosphorylation. This change towards glycolytic energy production (Warburg effect) under inflammatory conditions allows the quick generation of energy needed for the induction of an efficient immune response. Inflammation of the periodontal tissue is an osteolytic disease that has a bacterial onset characterised by biofilm formation of P. gingivalis, however it seems clear now that this does not necessarily lead to periodontal disease (PD). In fact, PD progression can be attributed to excess inflammation as a result of a non-resolving inflammatory reaction of the host immune system. Resolution is an active process that is triggered by a change in enzymatic fatty acid modifying proteins from pro-inflammatory lipid mediators such as prostaglandins (produced by COX) and leukotrienes towards specialised pro-resolving mediators” (SPM). SPMs bind to specific GPCRs to trigger intracellular signaling that causes termination of neutrophil infiltration, increased macrophage phagocytosis (efferocytosis) as well as tissue remodelling. In this project we investigate the impact of metabolic changes on inflammation and resolution, respectively.
Modell systems and Techniques:
Primary murine cells (DC, macrophages, osteoclasts), metabolic assays, western blots, RT-PCR, biochemical cell fractionation, FACS, NMR studies of metabolites (with Dr. Souto-Carneiro)
Selected Publications:
Kubatzky KF, Gao Y, Yu D
Post-translational modulation of cell signalling through protein succinylation.
Explor Target Antitumor Ther. 2023;4(6):1260-1285.
Kubatzky, KF, Uhle F., Eigenbrod T. 2018
From macrophage to osteoclast: How metabolism determines function and activity
Cytokine. 112:102-115.
Members:
Vincent Ebert
Franziska Kraus (Alumni)
Kathrin Schultheiß (Alumni)
Lab Members
| Lab members | phone +49 6221 56- | |
| Katharina Kubatzky | Group Leader | -38361 |
| Gabriele Sonnenmoser | Technician | -39414 |
| Elisabeth Seebach | PostDoc | -7801 / -4638 |
| Yue Gao | Master Student | -7804 / -7801 |
| Carlos Ernesto Pérez Cevallos | MD Student |
Im Neuenheimer Feld 324, Brücke zu 325
69120 Heidelberg
GERMANY
Phone +49 6221 56 38361
Fax +49 6221 56-33749
Twitter @kkubatzky
Funding
DAAD
DFG
Medizinische Fakultät Heidelberg
Ontario-Baden-Württemberg Exchange Program
Curriculum Vitae

Education:
- 2019: Faculty member of HBIGS graduate school, Heidelberg
- 2018: apl. Professorin, Ruprecht-Karls-University, Heidelberg (Germany)
- 2012: Habilitation in Molecular Medicine, Ruprecht-Karls-University, Heidelberg (Germany)
- 2011: Teaching Certificate "Baden-Württemberg Zertifikat für Hochschuldidaktik"
- 2001: PhD from the Albert-Ludwigs-University, Freiburg, and Max-Planck-Institute of Immunobiology, Freiburg (Germany)
- 1997: Studies in Chemistry (Diploma) at the Albert-Ludwigs-University, Freiburg (Germany)
Professional experience:
- Since 2012 permanent position as Group leader and Principal Investigator at the Department of Infectious Diseases, Medical Microbiology and Hygiene, Heidelberg (Germany)
- 2008: Visiting scientist at the Department of Pathology, University of Michigan, Ann Arbor (USA) on a Max-Kade Fellowship
- 2007-: Prinicipal Investigator at the Department of Infectious Diseases, Medical Microbiology and Hygiene, Heidelberg (Germany)
- 2005-6: Junior Group Leader at the Institute for Experimental and Clinical Pharmacology and Toxicology at the Albert-Ludwigs-University, Freiburg (Germany)
- 2002-4: Postdoctoral Fellow (Delori Postdoctoral Fellowship of the Christian de Duve Institute of Cellular Pathology), Ludwig Institute for Cancer Research, Brussels (Belgium)
- 2001-2: Research scientist at Alantos Pharmaceuticals, Heidelberg (Germany)
Awards and Fellowships:
- 2009-12: Olympia-Morata Habilitation Fellowship, University of Heidelberg
- 2008: Max Kade Fellowship for a one-year research stay in the USA
- 2002-4: Delori Postdoctoral Fellowship of the Christian de Duve Institute for Cellular Pathology (ICP) at the Université Catholique de Louvain, Belgium