Leistung
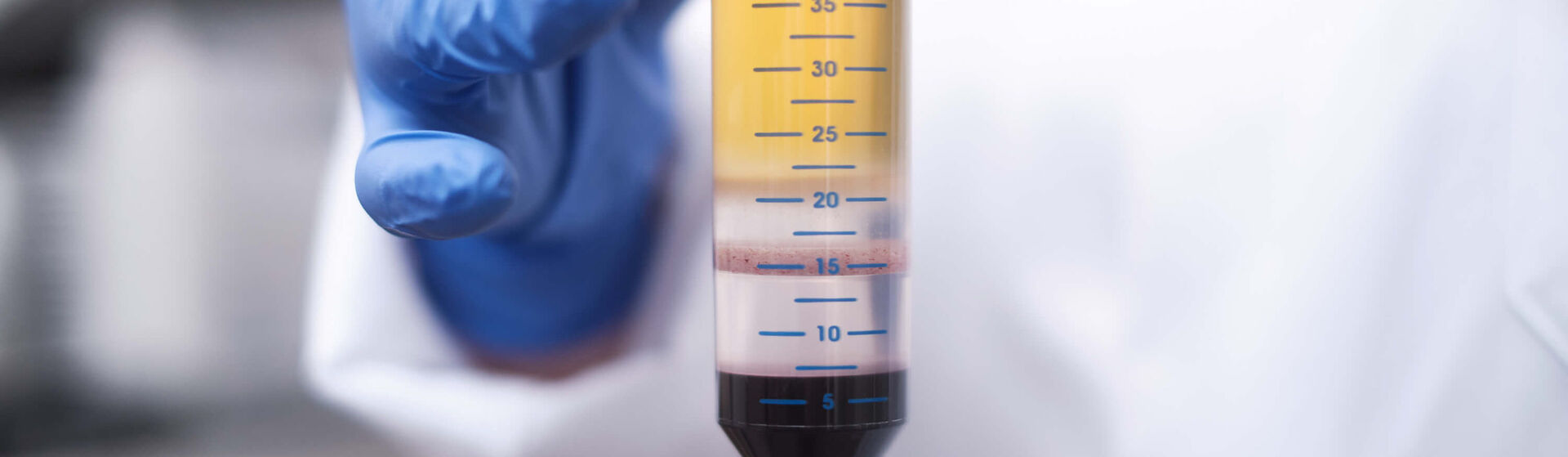
Directly after acceptance or delivery, vital peripheral mononuclear cells (PBMC) are isolated under sterile conditions and with high yield according to the respective requirements/lab manuals. The isolation is documented in compliance with GCP. The laboratory team is very familiar with isolations via Ficoll/Histopaque gradients but also via Vacutainer and Leucosep tubes as well as with microscopic counting of the cells. After controlled freezing, PBMC are stored either at -80 °C or in liquid nitrogen until collection/shipment. The laboratory is certified according to ISO 9001:2015.
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/5/6/csm_willkommen_d34eda0786.png)

![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/8/5/csm_ACL_11_GrafikFingerTablet_LB8_c4ce258375.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/3/a/csm_KS_b1_PatBett_Banner_719c5df562.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/6/4/csm_publikaitonen_4abdce50fc.png)




