Research areas
Our department has two main goals: On the one hand we promote the translation of new therapies into humans through early clinical trials (ECT) and the analysis and qualification of drugs in our laboratories, and on the other hand we maximize the medication safety of already approved therapies. We are supported in this endeavor by the key technologies and research expertise listed below, and by our dedicated, multidisciplinary team and experienced staff.
Focus Early Clinical Trials
Early clinical drug trials / initial applications (first-in-human studies)
Background: Clinical drug development begins with a first-in-human (FIH) trial. Conducted under maximum safety precautions, FIH trials reveal dose-dependent tolerability, pharmacokinetics, and sometimes also initial pharmacodynamic aspects. They determine which doses should be tested in the future. The development of 33% of all new drugs fail at this stage.
Experience: ~ 40 FIH studies in recent years.
Contact person: PD Antje Blank, MD
Department involved: Early Clinical Pharmacological Trial Unit
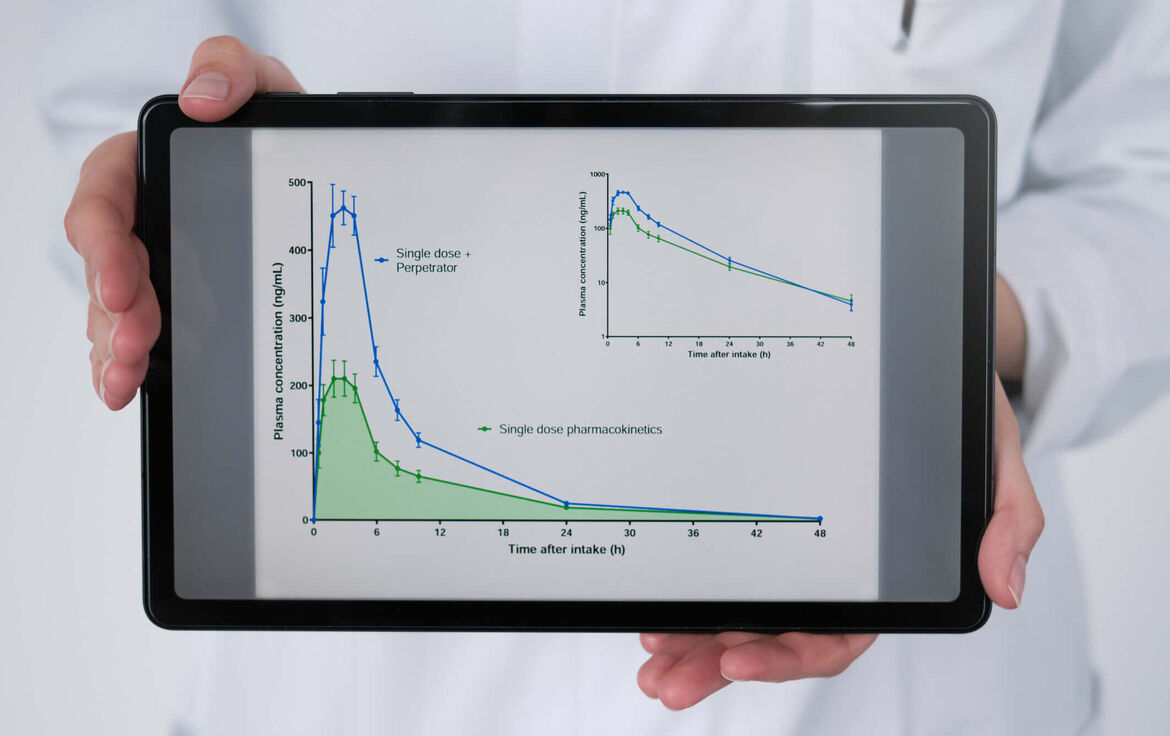
Qualitative and quantitative description of drug-drug interactions in vivo and in vitro
Background: Drug-drug interactions can cancel out their effects or amplify them into toxic ranges and therefore require dose adjustments or avoidance strategies. However, interactions may also be necessary for an effect to develop in the first place. Sound quantitative knowledge of drug interactions is therefore essential to combine drugs safely and effectively.
Experience: > 45 clinical DDI studies and > 40 DDI in vitro studies.
Contact person: PD Antje Blank, MD
Involved department areas:
Early Clinical Pharmacological Trial Unit Analytical-Chemical Laboratory Molecular Biological-Biochemical Laboratory Pharmacoepidemiology Drug Utilisation and Drug Safety
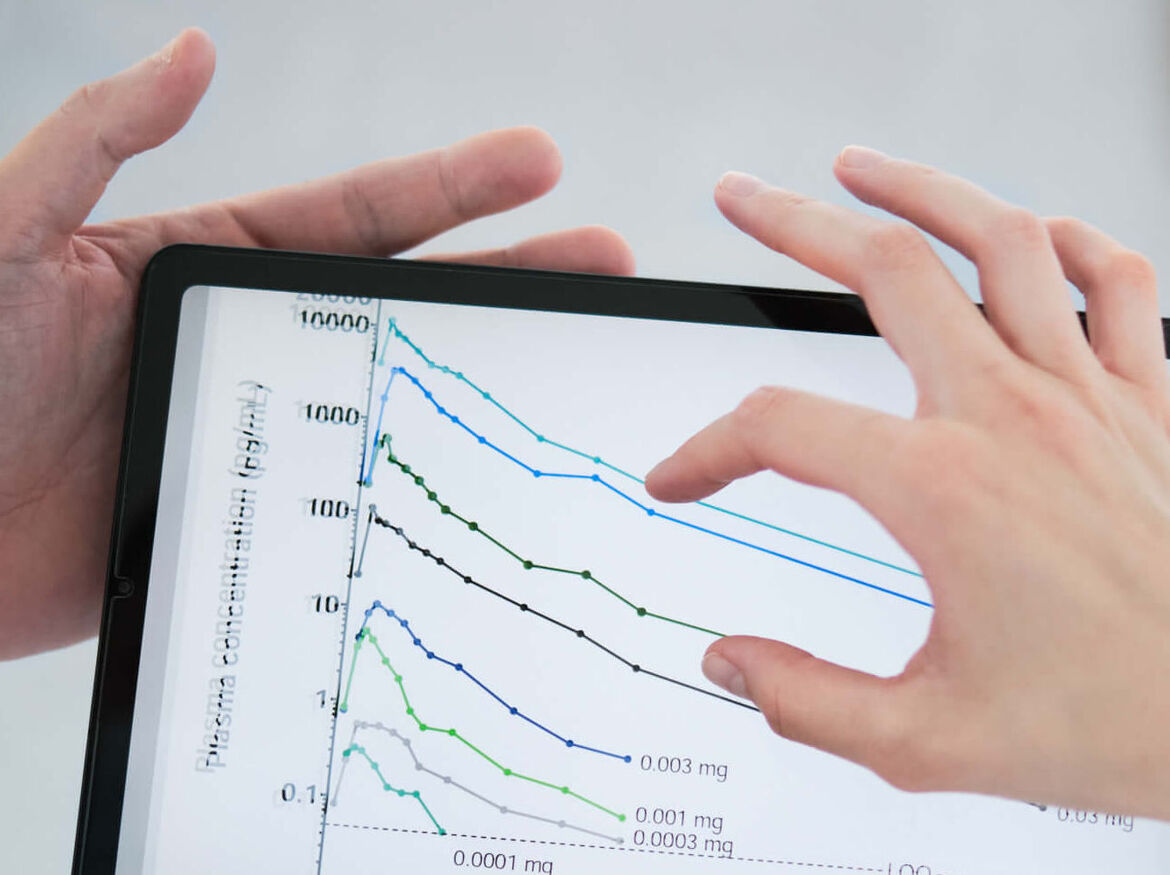
Microdosing
Background: With sufficient analytic sensitivity, drugs can be pharmacokinetically characterized even when administered at doses below the NOEL (No Observed Effect Level) or at a maximum of 1% of a therapeutic dose (typically < 100 ng). In addition, microdosed sacrificial substances can be used individually or as a cocktail to detect interactions with (normally dosed) perpetrator substances and thus can be used at low risk even in polypharmacy patients.
Experience: > 20 clinical studies with microdosed drugs, validated analytics for 7 microdosed test substrates.
Contact person: PD Antje Blank, MD
Involved department areas: Early Clinical Pharmacological Trial Unit Analytical Chemistry Laboratory
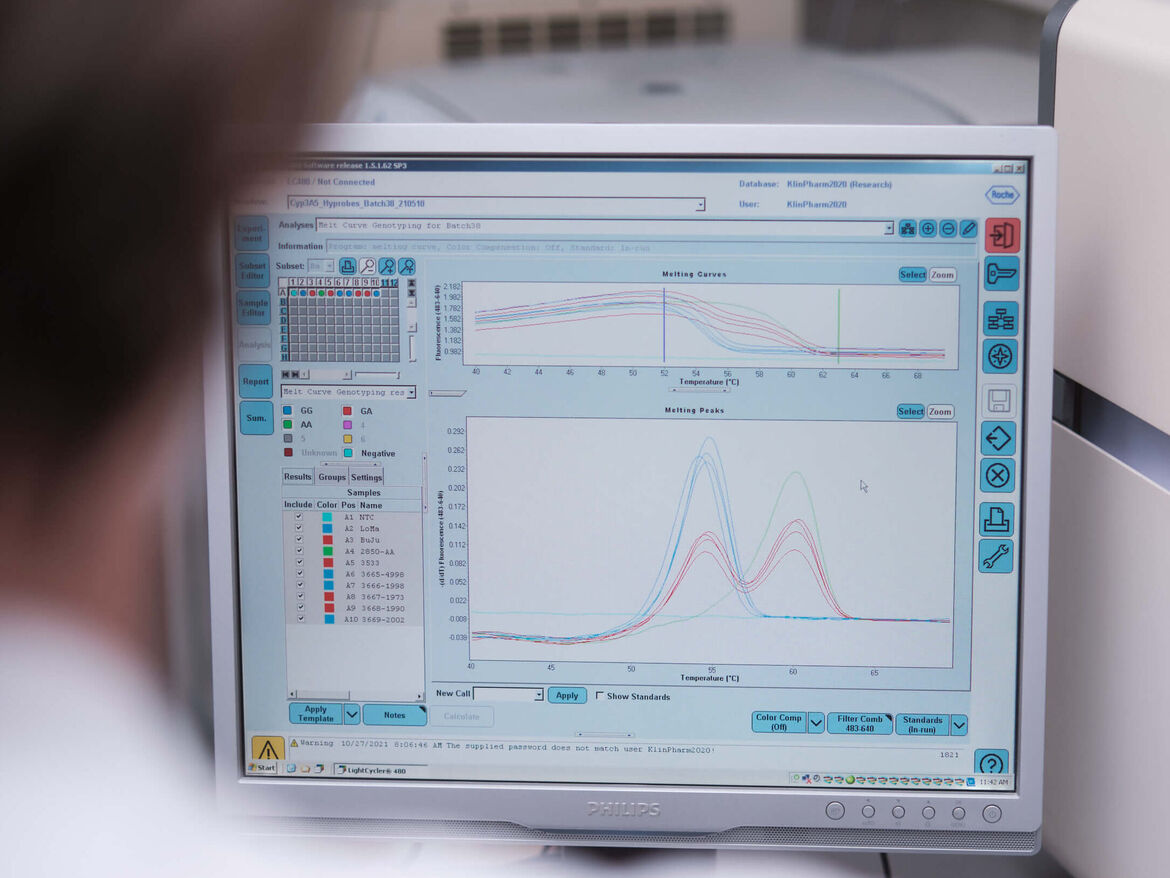
Influence of genetic polymorphisms on drug pharmacokinetics
Background: Genetic differences in drug-metabolizing enzymes or transporters can influence the pharmacokinetics of drugs as well as their interaction potential. All this can significantly alter the dose requirements of the individual patient and therefore be of clinical relevance.
Contact person: PD Antje Blank, MD
Involved department areas: Early Clinical Pharmacological Trial Unit Analytical Chemistry Laboratory Molecular Biology Biochemistry Laboratory
Pharmacokinetics and pharmacodynamics of immunosuppressants and in renal insufficiency
Background: Special patient populations (e.g. patients with renal failure, transplanted patients with immunosuppression) require highly individualized dosing to ensure efficacy and avoid toxicity. Therefore, it is important to understand influencing factors (e.g. interactions) and to further develop strategies to monitor and optimize individual therapy (tailored therapy).
Contact: Prof. Dr. med David Czock PD Antje Blank, MD
Involved department areas: Drug Utilisation and Drug Safety Early Clinical Pharmacological Trial Unit
Focus drug analysis and quantification
New dosage forms (oralization of peptides, 2D printing)
Background: To improve the oral bioavailability of peptide drugs and to circumvent long-term parenteral administration, we develop active absorption-enhancing excipients and test them in animal models and clinical studies.
In parallel, drugs with individually highly variable dosing requirements are printed on orodispersible films using 2-dimensional printing techniques. Their systemic availability and mucosal permeability are then tested in adults and children.
Experience: micro- and normal-dose orodispersible films, 1 new active excipient in development.
Contact person: Dr. Jürgen Burhenne
Departmental areas involved: Analytical Chemistry Laboratory Early Clinical Pharmacological Trial Unit
Highly sensitive drug quantification
Background: Validated analytical methods with the highest sensitivity (partly with femtomolar detection limits) are a prerequisite for the quantification of drugs after the administration of microdoses or in very small sample volumes. The latter can be samples from premature infants, small animals, dried blood samples (dried-blood-spots) or (microdissected) tissue samples.
Experience: Validated ultrasensitive analytics for 7 test substrates.
Contact person: Dr. Jürgen Burhenne
Department involved: Analytical Chemistry Laboratory
Intracellular pharmacokinetics and pharmacodynamics
Background: Plasma concentrations are often not the most suited surrogate for the effect of a drug; rather, the concentrations at the site of action, i.e. mostly in the target cell, are decisive. We are therefore investigating intracellular relationships between local concentrations and locally achieved or systemic effects.
Contact person: Prof. Dr. med. Johanna Weiß
Department involved: Molecular Biology-Biochemistry Laboratory
Focus medication safety
Strengthening patient competencies and promoting patient-centered medication safety
Background: For patients, taking medicines is often complicated, time-consuming and associated with side effects. Because the motivation and good adherence of patients is crucial for success, especially in long-term therapies, the implementation of one’s own therapy should be clear and correspond well to individual health needs. The goal of this research focus is to reliably ascertain both individual patient preferences and information needs and to address them accordingly.
Contact: Prof. Dr. sc. hum. Hanna M. Seidling
Department involved: Cooperation Unit Clinical Pharmacy
Interprofessional collaboration and measures to improve medication safety
Background: In any drug therapy, every single step of the medication process can become a hurdle to adherence and therefore turn into a risk. Often, multiple health professional groups are involved in the care process and their services need to be well coordinated, in parallel or consecutively. We conduct detailed on-site process analyses that provide a starting point for the selection, development, piloting, implementation and analysis of (often digital) medication safety measures. We also develop ways to optimize interprofessional collaboration and medication safety tools for inpatient and outpatient settings.
Contact: Prof. Dr. sc. hum. Hanna M. Seidling
Department involved: Cooperation Unit Clinical Pharmacy
Electronic decision support and knowledge transfer in medication safety
Background: Electronic information sources and decision support have revolutionized error prevention in the medication process. Timeliness, content quality and accuracy of warnings are core elements of good medication safety software. This software must also be as deeply as possible integrated into the process whose risks are to be minimized. For decades, we have been developing electronic tools for healthcare professionals and patients together with future users and take great care to pilot their suitability for everyday use before applying them in the field (e.g. in AiDKlinik). Avoiding over-alerting is an important goal in this context.
Contact person: Dipl.-Inform. Med. Michael Metzner
Department involved:
Medical Information Technology
Real-world evidence in large databases
Background: Efficacy (comparative effectiveness research) of specific therapeutic strategies is investigated using big data sources, such as routine data from health insurance companies. In addition, adverse drug reactions are analyzed, which also allow safety issues of low prevalence events or risks of drug combinations (interactions) to be investigated.
Contact person: PD Dr. sc. hum. Andreas Meid
Department involved: Pharmacoepidemiology
Pharmacometrics and Quantitative Systems Pharmacology
Background: Based on models of pharmacokinetics (population pharmacokinetics, physiology-based pharmacokinetics), drug concentrations and effects are modeled over time. In addition, possible therapeutic regimens are simulated, and, where appropriate, monitored using concentration- or biomarker measurements.
Contact person: PD Dr. sc. hum. Andreas Meid
Department involved: Pharmacoepidemiology
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/5/6/csm_willkommen_d34eda0786.png)

![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/8/5/csm_ACL_11_GrafikFingerTablet_LB8_c4ce258375.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/3/a/csm_KS_b1_PatBett_Banner_719c5df562.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/6/4/csm_publikaitonen_4abdce50fc.png)