Scope
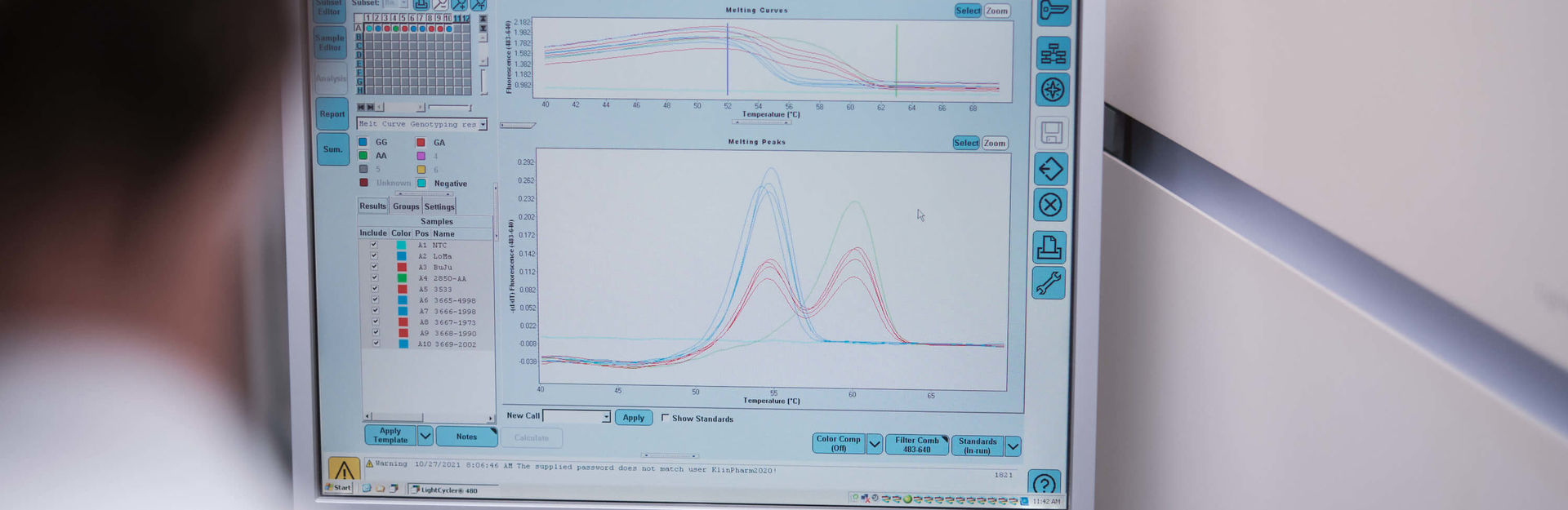
Pharmacogenetically relevant polymorphisms are determined within the framework of research collaborations. The methods used are all validated and based on LightCycler technology (melting curve analysis with hybridization probes or simple probes). New methods can also be established upon request. Whole blood samples, oral swabs or already isolated DNA are required as starting material. The laboratory is certified according to ISO 9001:2015.
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/5/6/csm_willkommen_d34eda0786.png)

![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/8/5/csm_ACL_11_GrafikFingerTablet_LB8_c4ce258375.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/3/a/csm_KS_b1_PatBett_Banner_719c5df562.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/6/4/csm_publikaitonen_4abdce50fc.png)




