Arbeitsgruppe Prof. Dr. Schäkel
Prof. Dr. med. Knut Schäkel
Leitender Oberarzt
(Hautklinik)
Arbeitsgruppenleiter
(AG Prof. Dr. med. K. Schäkel)
Research Focus
Our studies aim for a better understanding and treatment of chronic inflammatory skin diseases. Our basic science-oriented research laboratory is located in a modern university hospital. This allows for translational research starting with a clinical question that is addressed in our basic research lab and translated back to bedside.
We have been studying the role of leukocytes in the context of psoriasis, atopic dermatitis, lupus erythematodes and malignant melanoma.
Our current studies are specifically aiming for a better understanding of non-classical monocytes in skin inflammation, the role of immune complexes in inducing cell migration and the interplay of leukocytes and keratinocytes.
Projects
FUNCTIONAL SPECIALIZATION OF MONONUCLEAR PHAGOCYTES IN PSORIASIS
Our long-term interest is to study the role of mononuclear phagocytes (monocytes, macrophages and dendritic cells) in immune responses. These cells are present at high numbers in the skin as well as in other organs and are subdivided into populations with specific phenotypical and functional properties. We previously defined a distinct population of monocytes and identified their marker antigen as slan (6-sulfo LacNAc), which is expressed on the homing molecule P-selectin glycoprotein ligand 1 (PSGL-1). These cells belong to the population of non-classical monocytes.
SlanMo produce the T cell stimulating cytokines IL-12, IL-23, TNF-α and IL-1ß at high levels and induce a strong Th1/Th17 T cell response. In line with these functional features we identified slanMo as inflammatory dermal cells in psoriasis, atopic dermatitis and lupus erythematosus. In addition, current studies indicate a functional role for slanMo in human cancer lymph-node metastasis, multiple sclerosis, Crohn's disease and viremic HIV infection.
The expression of Toll-like receptors (TLR) 4, 7 and 8 accounts for their low activation threshold and their proinflammatory potential in autoimmune diseases. Other functional features of slanMo are their outstanding capacity to bind immune complexes via the low affinity Fcγ receptor IIIa (CD16a).
With specific mouse models we are currently studying the in vivo function of non-classical monocytes
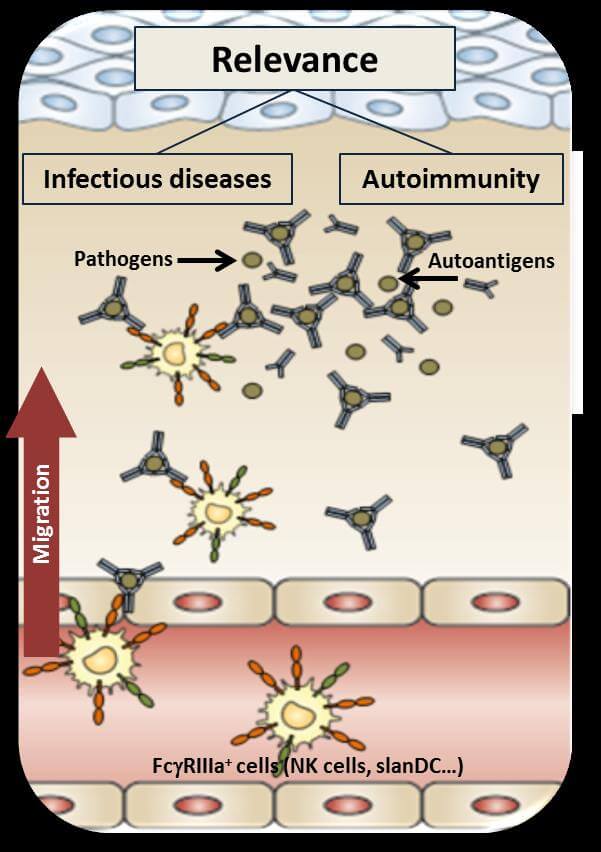
IMMUNE COMPLEX MEDIATED RECRUITMENT OF LEUKOCYTES
Immunoglobulin G (IgG) is important for the antimicrobial immune defense, can cause autoimmune diseases and is successfully used for therapeutic purposes. Understanding of the IgG-mediated immune defense is of great medical and scientific interest. We study the novel role of IgG immune complexes in cell migration. We previously reported on the local vascular recruitment of human slan+ non-classical monocytes (slanMo) from blood circulation via IgG immune complexes interacting with low affinity Fcγ receptor III. We demonstrated that slanMo serve as the dominant pro-inflammatory cell type in early immune complex-induced lupus nephritis. Immune complexes were shown to recruit and direct a first wave of immune cells that may prove essential for controlling early microbial infection in a seropositive host or for initiating IgG-dependent autoimmune inflammation. These studies explore the functional role of IgG-immune complexes in cell recruitment and add to our understanding of IgG in immune defense. Results of our studies will provide knowledge with the potential for developing treatment strategies aiming at inhibiting immune complex-driven autoimmune diseases, stimulating pathogen-specific or cancer-directed immune responses.
Group members
Doktoranden/-innen
-

Qian Chen, M. Sc.
-

Michael Hertwig
-

Paul Dong-Huhn Lee
-

Amelie List
-

Romy Ludwig
Postdocs
-

Thomas Döbel (PhD)
Technischer Assistent
-

Stefan Meisel
Alumni
Ehemalige Kollegen
-

PhD Fareed Ahmad
-

Jing Cui
-

Felix Funck
-

Galina Gräbe
(Technician)
-

Anja Kunze
-

Michael Maas, PhD
-

Florina Olaru
-

Silvia Pezer
-

Hridaydesh Prakash (Phd)
-

Dr. med. Adriana Rendon Medina
-

Hao Zhang

Selected publications
- Freund L, Oehrl S, Schwingen J, Haeberle S, Döbel T, D.H. Lee P, Meisel S, Mihalceanu S, Rußwurm M, Luft T, Schäkel K.
IFNγ causes keratinocyte necroptosis in acute graft-versus-host disease. J Invest Dermatol. 2023 Mar 6:S0022-202X(23)00174-4. doi: 10.1016/j.jid.2023.02.025 - Preuß SL, Oehrl S, Zhang H, Döbel T, Engel U, Young JL, Spatz JP, Schäkel K.
Immune complex-induced haptokinesis in human non-classical monocytes. Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1078241 - Cui J, Oehrl S, Ahmad F, Brenner T, Uhle F, Nusshag C, Rupp C, Funck F, Meisel S, Weigand MA, Morath C, Schäkel K.
Detection of In Vivo Inflammasome Activation for Predicting Sepsis Mortality. Front. Immunol., February 2021 doi.org/10.3389/fimmu.2020.613745. - Funck F, Pahl J, Kyjacova L, Freund L, Oehrl S, Gräbe G, Pezer S, Hassel JC, Sleeman J, Cerwenka H, Schäkel K.
Human innate immune cell crosstalk induces melanoma cell senescence. Oncoimmunology. 2020 Aug 30;9(1):1808424. doi: 10.1080/2162402X.2020.1808424. - Freund L, Oehrl S, Gräbe G, Gholam P, Plum T, Schäkel K. Skin-selective CD8 T cell depletion by photoimmunotherapy inhibits human cutaneous acute graft- versus-host disease. J Invest Dermatol. 2020 Jul;140(7):1455-1459.e6. doi: 10.1016/j.jid.2019.12.009.
- Olaru F, Döbel T, Lonsdorf AS, Oehrl S, Maas M, Enk AH, Schmitz M, Gröne EF, Gröne H-J, Schäkel K. Intracapillary immune complexes recruit and activate slan-expressing CD16+ monocytes in human lupus nephritis. JCI Insight. 2018 Jun 7;3(11).
- Döbel T, Kunze A, Babatz J, Tränkner K, Ludwig A, Schmitz M, Enk A, Schäkel K: FcγRIII (CD16) equips immature 6-sulfo LacNAc-expressing dendritic cells (slanDCs) with a unique capacity to handle IgG-complexed antigens. Blood. 2013 (18):3609-18.
- Hänsel A, Günther C, Ingwersen J, Starke J, Schmitz M, Bachmann M, Meurer M, Rieber EP, Schäkel K: Human slan (6-sulfo LacNAc) dendritic cells are
inflammatory dermal dendritic cells in psoriasis and drive strong TH 17/TH 1 T-cell responses. J. Allergy Clin. Immunol. 2011 (127):787-794. - Schäkel K, von Kietzell M, Hänsel A, Ebling A, Schulze L, Haase M, Semmler C, Sarfati M, Barclay NO, Randolph GJ, Meurer M, Rieber EP. Human 6-sulfo
LacNAc-expressing dendritic cells are principal producers of early interleukin-12 and are controlled by erythrocytes. Immunity. 2006 (24):767-777. - Schäkel K, Kannagi R, Goto Y, Mitsuaoka C, Zwirner J, Soruri A, Kniep B, Rieber EP. 6-sulfoLacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity. 2002 (17) 289-301.