Scope
- Consulting for preclinical projects aiming at translation
- Development of modern, guideline-compliant and feasible trial designs for first-in-human (fih) projects
- Careful development and elaboration of trial protocol and participant/patient information according to regulatory requirements
- Support for EMA/BfArM/PEI scientific advice meetings
- Calculation of safe starting doses
Mission
We support the translation of new compounds with our clinical expertise. We ensure valid results through competent planning.
Background
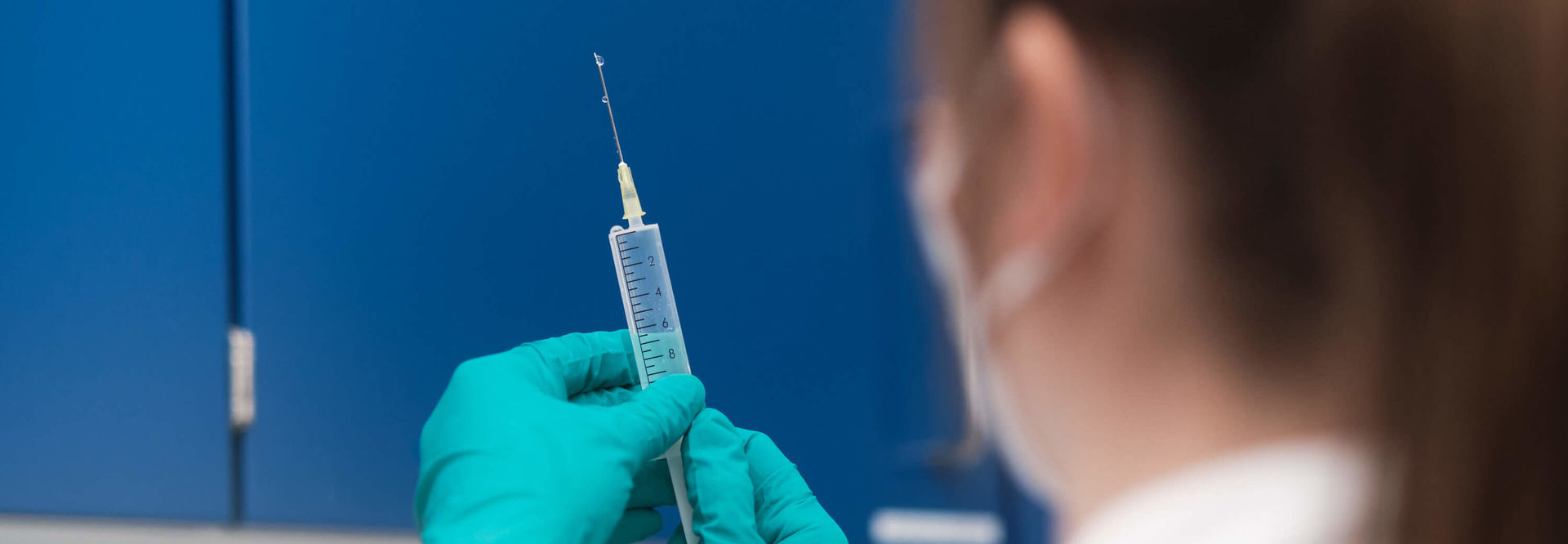
TTranslation, i. e. the implementation of preclinical results into a first-in-human clinical trial, is a crucial process and requires the cooperation of many disciplines. With our pharmacological expertise, we support the planning of this decisive step in the drug development process. Based on our experience from a multitude of first-in-human projects, we contribute to the design of GCP- and guideline-compliant translational trials and thus ensure a practical and safe trial implementation.
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/5/6/csm_willkommen_d34eda0786.png)

![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/8/5/csm_ACL_11_GrafikFingerTablet_LB8_c4ce258375.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/3/a/csm_KS_b1_PatBett_Banner_719c5df562.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/6/4/csm_publikaitonen_4abdce50fc.png)




