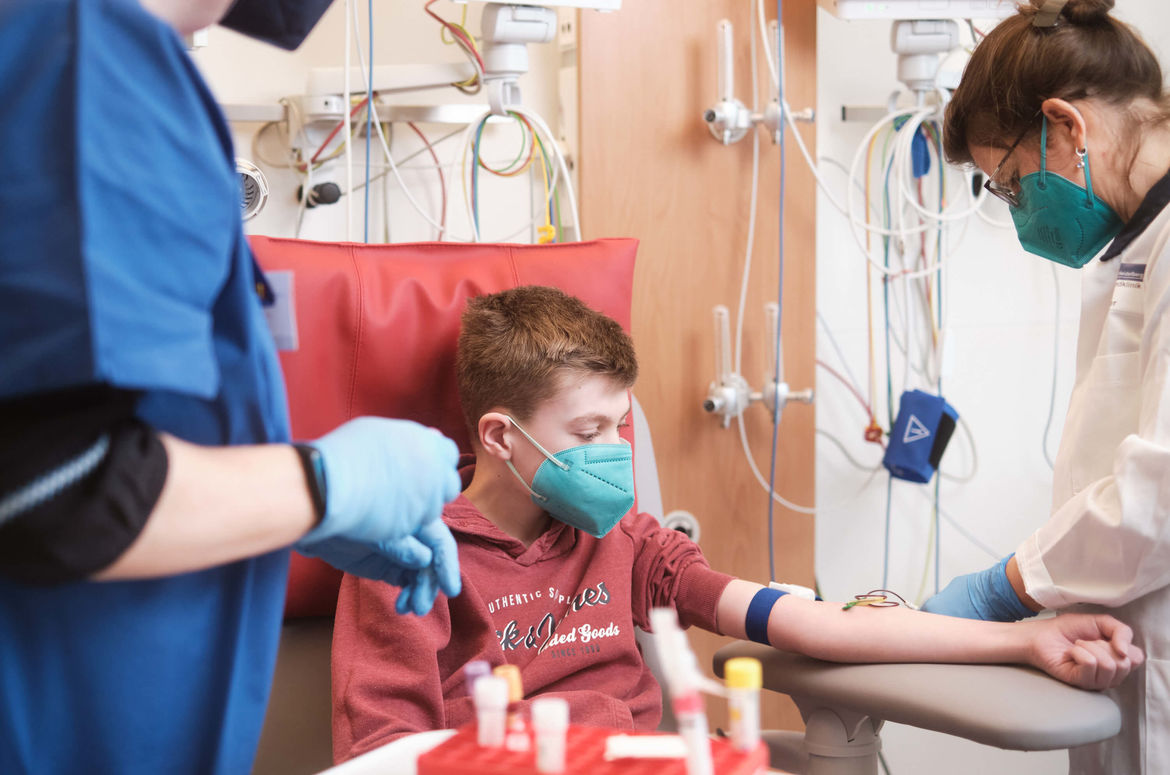
Services
- Planning and conduct of outpatient and (partially) inpatient study visits in accordance with trial protocol requirements, including 24-hour monitoring of study participants (if necessary over several days).
- Sampling, processing, shipping or temporary storage of biological samples in specialized, fully equipped facilities in compliance with all (inter)national regulatory requirements.
- GCP-compliant storage and management of study medication in dedicated refrigerators as well as room-temperature cabinets connected to the 24/7 central alarm control and monitoring system of the Heidelberg University Hospital, including a 24/7 continuous temperature monitoring.
- Organization and coordination of protocol-compliant storage, management and preparation of study medication also in the Pharmacy Department of the Heidelberg University Hospital with a protocol-compliant rapid delivery to our trial center for administration.
- GCP-compliant storage and management of study equipment in our trial center.
Mission
Our excellent modern study infrastructure enables high-quality GCP-compliant trial to be conducted at the highest safety and comfort levels.
Background
Our fully-equipped specialized phase 1 infrastructure (4 beds) with close access to the Intensive Care Unit (ICU) of the Center for Child and Adolescent Medicine (CCAM) guarantees a high level of patient safety and adequate patient care in both outpatient and inpatient trial settings. This includes conduct of extensive 24-hour pharmacokinetic studies as well as appropriate biological sample management. Immobile patients of all age groups, including premature newborns, can be involved in clinical trials under thorough multidisciplinary care using our decentralized satellite rooms in the Pediatric ICU, as well.