Projects of the working group
Introduction
![[Translate to English:] Modell eines Kaliumkanals in der Zellmembran.](/fileadmin/_processed_/b/c/csm_abb_1_e138262438.gif)
Cardiac arrhythmias are commonly encountered in clinical practice. They can be harmless, but they may also lead to life-threatening situations, especially if comcomitant heart diseases are present. Sudden cardiac death caused by cardiac arrhythmias is responsible for 15-20% of all natural deaths in Western countries. In the future, new, causal treatment concepts are to be developed through the identification ofmolecular mechanisms underlying cardiac arrhythmias (translational science).
Mutation analysis of patients with hereditary heart rhythm disturbances
![Mutation analyis [Translate to English:] Mutationsanalyse.](/fileadmin/_processed_/9/5/csm_MUTATION_5f8ce92ace.gif)
Patients with a familial form of ventricular arrhythmia and with sporadic occurrence of life-threatening cardiac arrhythmias are usually invasively examined to determine primary causes (coronary heart disease, cardiomyopathies) using diagnostic coronary angiography. For further clarification, invasive electrophysiological examinations are often also performed to provide information on the genesis and prognosis of the arrhythmia. In the course of a screening of patients we were able to identify mutations in ion channel genes, some of which have not been described so far. For analysis, genomic DNA was isolated from blood samples of patients and the exon sequences of important ion channel genes were checked by PCR and SSCP analysis. The amplified PCR products with abnormalities were isolated and checked by DNA sequence analysis after cloning of the fragments. In parallel, the cDNAs of relevant ion channels (KCNQ1, KCNH2, KCNE1, KCNE2) required for functional analysis of the mutations were isolated from human cDNA, sequenced and cloned into expression vectors. The mutated channels can then be expressed in cell systems and electrophysiologically characterized.
Blockage of cardiac potassium channels - Efficient antiarrhythmic therapy with potential risk
![[Translate to English:] Blockade kardialer hERG-Kaliumkanäle nach Applikation eines Zytostatikums. Die Ionenkanäle wurden durch das skizzierte Spannungsprotokoll aktiviert.](/fileadmin/_processed_/b/b/csm_abb_6_e389d69c55.gif)
Inhibition of the cardiac IKr (hERG) potassium current is the aim of therapy with class III antiarrhythmics. However, excessive inhibition of this current can lead to the acquired Long QT syndrome and result in sudden cardiac death. This disease is not only known as a side effect of therapy with class III antiarrhythmics, but has also been shown to be an undesirable effect of non-antiarrhythmic substances (e.g. psychotropic drugs, antibiotics, cytostatics). In this project, data on the chemical and biophysical properties of drugs responsible for acquired Long QT syndrome will be collected on the cloned hERG potassium channel. They are the basis for preclinical in vitro and in silico test procedures for risk evaluation in pharmaceutical research and development, which we are developing in cooperation with the Karlsruhe Institute of Technology.
Antiarrhythmic gene therapy
The therapy of cardiac arrhythmias with drugs, ablation procedures, pacemakers or defibrillators is insufficient in some cases with complex arrhythmias. For these cases we are investigating the effectiveness of gene therapy approaches in translational approaches. We are currently focusing our work on atrial fibrillation, the most common of all persistent cardiac arrhythmias, and on ventricular tachycardia after myocardial infarction.
Cardiac pacemaker channels
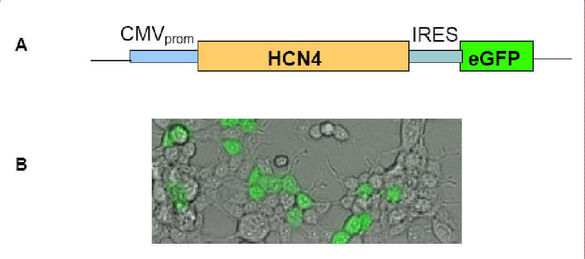
Sinus node dysfunction is the most common indication for the implantation of an electric pacemaker. Gene therapy and cell transplantation represent current therapeutic concepts for the restitution of cardiac dysfunction. One of our goals is to establish a biological pacemaker system from genetically modified mesenchymal stem cells (MSC), which should come significantly closer to physiological sinus node function than electrical pacemaker systems. For this purpose, adult MSC will be used which, after viral transduction, express the pacemaker channel HCN4, which is the molecular correlate of the "funny" current (If) responsible for pacemaker activity in the sinus node. In a second approach, MSCs will be stimulated "in vitro" for cardiomyogenic differentiation to generate pacemaker-like cells.
In addition, we have identified different mutations in the HCN4 gene in patients with sinus node dysfunction without structural heart disease using SSCP analysis. It is of great interest to functionally characterize and describe the new mutations. We have therefore cloned the mutations occurring in different exons of the HCN4 gene in plasmid vectors and identified them genetically by DNA sequence analysis. For exact characterization we perform electrophysiological measurements after transfection and expression in HEK cells.
Proteomic analysis of macromolecular ion channel complexes in the heart
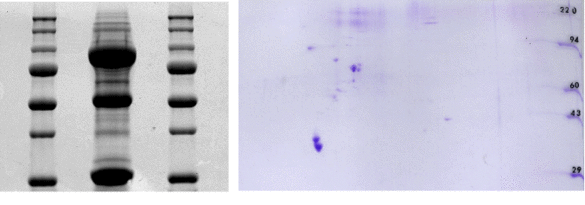
Current findings from proteomics show that the regulation of cardiac ion channels and thus the control of the heart rhythm cannot be attributed to the channel protein itself in isolation. Rather, arrhythmogenesis is regarded as a multifactorial event, with macromolecular protein complexes at its centre. These are composed of the ion channel protein, accessory beta-subunits, protein kinases and phosphatases as well as adapter, anchoring and signal transduction molecules. Using proteomic methods such as pull-down assays and two-dimensional gel electrophoresis (see figure), yeast-two-hybrid screening and mass spectrometry, we identify new components of protein complexes in the heart. The function of the newly identified proteins is characterized using electrophysiological techniques (patch clamp, double electrode voltage clamp).
Antiarrhythmic therapy by modulation of two-pore domain potassium channels
The complex regulation of the two-pore domain potassium channels (K2P) makes a decisive contribution to the plasticity of cardiac excitability and offers valuable therapeutic targets. An increase in their conductivity has the effect of lowering the membrane potential below the action potential threshold and reducing the proarrhythmic potential in the heart. This project aims to characterize the modulation of cardiac K2P channels by protein kinases and to decipher intracellular signal transduction mechanisms in detail.

![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/2/3/csm_20210614_MedKlinik_004_26a0b1fc8e.jpg)