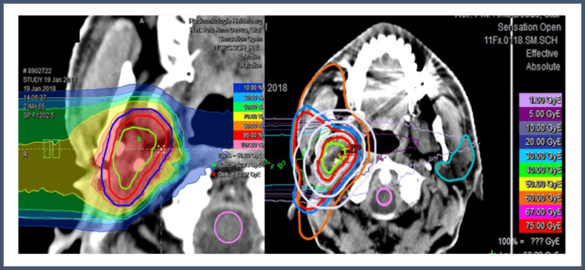
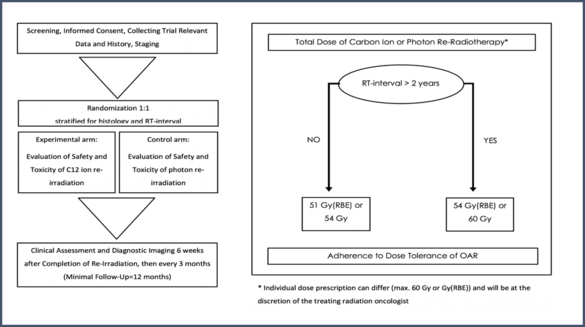
Carbon Ion Re-Radiotherapy in Patients with Recurrent or Progressive Locally Advanced Head-and-Neck Cancer: A Phase-II Study to Evaluate Toxicity and Efficacy
Zusammenfassung
Die Studie untersucht Kohlenstoff Ion Re-Radiotherapie bei Patienten mit rezidivierenden Kopf-Hals-Tumoren im Vergleich zu einer Intensitätsmodulierten-Radiotherapie (IMRT). Die Studien beinhaltet eine breites translationales Program mit Bestimmung der zirkulierenden Tumor-DNA im longitudinalen Verlauf, als auch die prospektive Auswertung bildgebender MRT Parameter.
Einschlusskriterien
- Locally recurrent / progressive head-and-neck cancer after initial radiation therapy
- Microscopic or macroscopic tumor after salvage surgery
- Indication for re-irradiation
- Completed wound healing after surgical intervention
- Karnofsky-Performance-Score ≥ 60
- Age ≥ 18 years
- Written informed consent (must be available before enrolment in the trial)
- Ability of subject to understand character and individual consequences of the trial
- For women with childbearing potential, (and men) adequate contraception
- Submission of previous radiotherapy records
Ausschlusskriterien
- Re-irradiation of malignancy in the larynx
- Diagnosed plasymocytoma, sarcoma or chordoma
- Previous re-irradiation in-field
- Time interval < 6 months after initial radiotherapy
- Distant metastases (except pulmonary metastases)
- Patients who have not recovered from acute toxicities of prior therapies
- Refusal of the patients to take part in the study
- Pregnant or lactating women
- Known carcinoma <5 years ago (excluding Carcinoma in situ of the cervix, basal cell carcinoma, squamous cell carcinoma of the skin) requiring immediate treatment interfering with study therapy
- Participation in another clinical study or observation period of competing trials, respectively
- Re-irradiation of malignancy in the larynx