MRD Laboratory
Requisition Slip for MRD Diagnostics (pdf)
Acute lymphoblastic leukemia (ALL) ist the most frequent of all malignant diseases in childhood, accounting for 30-35% of all cases. The use of intensive and consistently timed polychemotherapy regimens leads to a complete clinical remission in 98% of affected children within four weeks after starting treatment, i.e. leukemia cell counts fall below the detection limits of conventional diagnostic methods (< 1-5% of malignant cells in the bone marrow). However, a reduction of the malignant cell population by two powers of ten means that a significant amount/proportion of residual leukemic cells may still prevail, but not be detectable by conventional methods (i.e. morphology, imunophenotyping, cytogenetics and Southern blot analysis).
The use of quantitative "real-time" PCR-technology (RQ-PCR) allows for a much more sensitive detection of minimal residual leukemia cells (mimimal residual disease, MRD), such that analysis of appropriate DNA markers typically detects one in ten thousand (10-4) leukemia cells or even one in one-hundred thousand (10-5) normal lymphocytes. As a marker-system, we use clone-specific immunoglobulin (Ig) and T-cell receptor (TCR) gene arrays and ideally employ two different gene probes with a detection limit of ≤ 10-4 for each patient.
Consequently, molecular diagnostic detection of minimal residual disease by RQ-PCR is of high prognostic value for recurrence risks in patients and risk-adapted therapies.
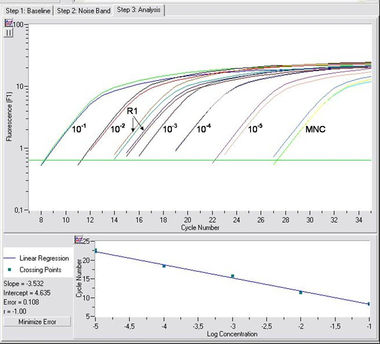
Figure: RQ-PCR experiment of an IgH rearrangement (by use of probe) with a detection limit of 10-5 and measurement of a time point of therapy (R1); MNC (mononuclear cell background).
Our Team
Laboratory Director
-

Dr. phil. nat. Rolf Köhler
Focus
Leukemia and MRD diagnostics
Medical-technical assistants
Medical-technical laboratory assistants
Alumni
Scientists:
Dr. T. Seriu
Dr. M. Nakao
Dr. T. Flohr
MTAs:
Simone Busenbender
Kirsten Linsmeier
Cornelia Rütz
Ivonne Schmitt
Yvonne Yeboah
Heike Kuzan
Daniela Siragusa
Nicole Schatz
Cooperations
Prof. Dr. M. Schrappe (ALL-BFM-Studie 95 + 2000) Klinik für Allgemeine Pädiatrie, Universität Kiel
Prof. Dr. D. Hölzer (GMALL 06/99+ 07/03-Studie) Med. Klinik II, Universität Frankfurt
Prof. Dr. M. Kneba (GMALL 06/99+ 07/03-Studie) Med. Klinik II, Universität Kiel
ESG-MRD-ALL (European Study Group on Minimal Residual Disease Detection in Childhood acute lymphoblastic Leukaemia)
Links
www.kinderkrebsinfo.de/erkrankungen/leukaemien
www.kompetenznetz-leukaemie.de
Selected Publications
van Dongen JJ, Seriu T, Panzer-Grumayer ER, Biondi A, Pongers-Willemse MJ, Corral L, Stolz F, Schrappe M, Masera G, Kamps WA, Gadner H, van Wering ER, Ludwig WD, Basso G, de Bruijn MA, Cazzaniga G, Hettinger K, van der Does-van den Berg A, Hop WC, Riehm H, Bartram CR: Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998 352: 1731
Pongers-Willemse M.J., Seriu T., Stolz F., Corral L., Gameiro P., Pisa P., Gonzales M., Bartram C.R., Panzer-Grümayer E.R., Biondi A., San Miguel J.F., van Dongen J.J.M.: Primers and protocols for standardized detection of minimal residual disease in acute lymphoblastic leukemia using immunoglobulin and T cell receptor gene rearrangements and TAL1 deletions as PCR targets. Leukemia 13, 110-118, 1999.
Nakao M., Janssen J.W.G., Flohr T., Bartram C.R.: Rapid and reliable quantification of minimal residual disease in acute lymphoblastic leukemia using rearranged immunoglobulin and T-cell receptor loci by LightCycler technology. Cancer Res. 60, 3281-3289, 2000.
Biondi A., Valsecchi M.G., Seriu T., D’Aniello E., Willemse M.P., Fasching K., Pannunzio A., Gadner H., Schrappe M., Kamps W.A., Bartram C.R., van Dongen J.J.M., Panzer-Grümayer E.R.: Molecular detection of minimal residual disease is a strong predictive factor of relapse in childhood B-lineage acute lymphoblastic leukemia with medium risk features. A case control study of the International BFM-Study Group. Leukemia 14, 1939-1943, 2000.
Willemse M.J., Seriu T., d'Aniello E., Hop W.C.J., Panzer-Grümayer E.R., Biondi A., Schrappe M., Kamps W.A., Masera G., Gadner H., Riehm H., Bartram C.R., van Dongen J.J.M.: Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor-B-ALL. Blood 99, 4386-4393, 2002.
Brüggemann M, Raff T, Flohr T, Gökbuget N, Nakao M, Droese J, Lüschen S, Pott C, Ritgen M, Scheuring U, Horst HA, Thiel E, Hoelzer D, Bartram CR, Kneba M; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006 Feb 1;107(3):1116-23.
van der Velden VH, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grumayer ER, Flohr T, Sutton R, Cave H, Madsen HO, Cayuela JM, Trka J, Eckert C, Foroni L, Zur Stadt U, Beldjord K, Raff T, van der Schoot CE, van Dongen JJ; European Study Group on MRD detection in ALL (ESG-MRD-ALL). Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007 Apr;21(4):604-11.
Flohr T., Schrauder A., Cazzaniga G., Panzer-Grümeyer R., van der Velden V., Fischer S., Stanulla M., Basso G., Niggli F.K., Schäfer B., Sutton R., Koehler R., Zimmermann M., Valsecchi M.G., Gadner H., Masera G., Schrappe M., van Dongen J.J.M., Biondi A., Bartram C.R.: Minimal residual disease – directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP – BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia 22, 771-782, 2008
Schwarz A.K., Stanulla M., Cario G., Flohr T., Sutton R., Möricke A., Anker P., Stroun M., Welte K., Bartram C.R., Schrappe M., Schrauder A.: Quantification of free total plasma DNA and minimal residual disease detection in the plasma of children with acute lymphoblastic leukemia (ALL). Ann. Hematol. 88, 897-905, 2009
Conter V.*, Bartram C.R.*, Valsecchi M.G., Schrauder A., Panzer-Grümayer R., Möricke A., Arico M., Zimmermann M., Mann G., De Rossi G., Stanulla M., Locatelli F., Basso G., Niggli F., Barisone E., Henze G., Ludwig W.D., Haas O.A., Cazzaniga G., Koehler R., Silvestri D., Bradtke J., Parasole R., Beier R., van Dongen J.J.M., Masera G., Biondi A., Schrappe M.: Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia (ALL): results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood (in press) 2010
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/8/2/csm_20131204_Beratung_035_a396c6c6e5.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/a/0/csm_20170627_PflegeOrtho_001_fb912471fa.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/f/c/csm_20170215_LaborOMZ_155_c0169c0898.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/2/c/csm_20180523_Labor_139_6ebb9a0a1b.jpg)
