Determining the epidemiological parameters of Covid-19 through a household transmission study in a rural area of South Africa and Kenya
Acronym: COREP
Coordinator: Prof. Till Winfred Bärnighausen
Funding: The European & Developing Countries Clinical Trials Partnership (EDCTP; Grant RIA2020EF-3026)
Participating Organisations:
Fundación Privada Instituto de Salud Global Barcelona (ISGLobal), Spain
The Aga Khan University (AKU) - Kenya, Kenya
Department of Health, Kilifi County-Kenya, Kenya
Wits Reproductive Health and HIV Institute (WRHI), South Africa
Burnet Institute, Australia
University of Washington, United States


List of the other investigators
- Prof. Stanley Luchters, Aga Khan University, Nairobi, Kenya.
- Prof. Matthew F. Chersich, Wits Reproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand, South Africa.
- Prof Gijs Walraven, Aga Khan Development Network
- Prof. Emilio Letang, Fundación Privada Instituto de Salud Global Barcelona (ISGLobal), Spain
- Professor David Anderson, Burnet Institute, Australia.
- Prof. Jennifer Balkus, University of Washington, United States.
- A/Prof Anthony Ngugi, Aga Khan University, Nairobi, Kenya.
- A/Prof Shaheen Sayed, Aga Khan University, Nairobi, Kenya.
- Dr Gloria Maimela, Wits Reproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand, South Africa.
- Dr Alain Vandormael, Heidelberg University Medical School, Germany.
- Dr Julia Katherine Rohr, Harvard University, United States.
- Mrs Evaline Lang'at, Department of Health, Kilifi County, Kenya.
Research team members
- Dr Maureen Kimani, Kenya Ministry of Health, Department of Community Health Sciences, Kenya
- Dr John Kiiru, Kenya Ministry of Health, Department of Laboratory Sciences, Kenya
- Dr Francis Ndungu, KEMRI Kilifi, Kenya
- Mr Ben Kitole, Kilifi County Health Department, Kenya
- Mrs Caroline Gichuki, Aga Khan University, Kenya
- Mrs Eunice Irungu, Aga Khan University, Kenya
- Ms. Joy Mauti, Heidelberg University, Germany
- Dr Catherine Martin, Wits Reproductive and HIV Institute, Faculty of Health Sciences, University of Witwatersrand
- Ms. Dieyna Drame, Harvard University, USA
Summary
The epidemiological parameters of SARS-CoV-2 infection in sub-Saharan Africa are largely unknown and require population-, and household- and individual-level enquiry. Similarly, strategies for decentralised diagnostic testing for SARS-CoV-2 need adaptation and evaluation. This project addressed research gaps in the natural history and epidemiological parameters of SARS-CoV-2 infection using serologic-surveillance methodologies and household-cluster investigations, as per WHO COVID-19 study protocols. Secondarily, the project aimed to identify efficient, feasible, cost-effective approaches to the decentralized diagnosis of SARS-CoV-2 infection in rural areas. Finally, managing topics around risk factors infection and transmission during the implementation of the project anecdotal vaccine uptake and hesitancy were observed.
The Kenya household transmission study enrolled 11 households (4 from Kaloleni, 1 from Rabai, and 6 from Kilifi North). Two households withdrew from the study after visit 1 (6 participants). In total, 47 participants were enrolled (11 primary cases and 36 household contacts, of which 15 were children under 15 years of age. Of the 11 households, 5 households had PCR confirmed co-primary cases, and all 11 households had co-primary cases based on antibody testing. Of 36 household contacts, eight were PCR and antibody negative, of whom 1 became antibody positive at 7 of follow-up, though remained PCR negative. No other incidence cases were detected.The Kenya serial sero-surveillance study showed an increase in SARS-COV2 infection (based on IgG antibody assessments) over the period of time investigated. Data was analysed from the five rounds (each round 6 weeks separated from the other), with Round 1 (baseline): 1351 participants; round 2 1148participant); round 3 340 participants, round 4 820 participants, and round 5 1270 participants. Seroprevalence for IgG SARS-CoV-2 increased from 12,36% at Round 1 to 59.98% at the final visit six month later (round 5; Figure 1).
Figure 1: Sero-surveillance by individuals in Kenya Site
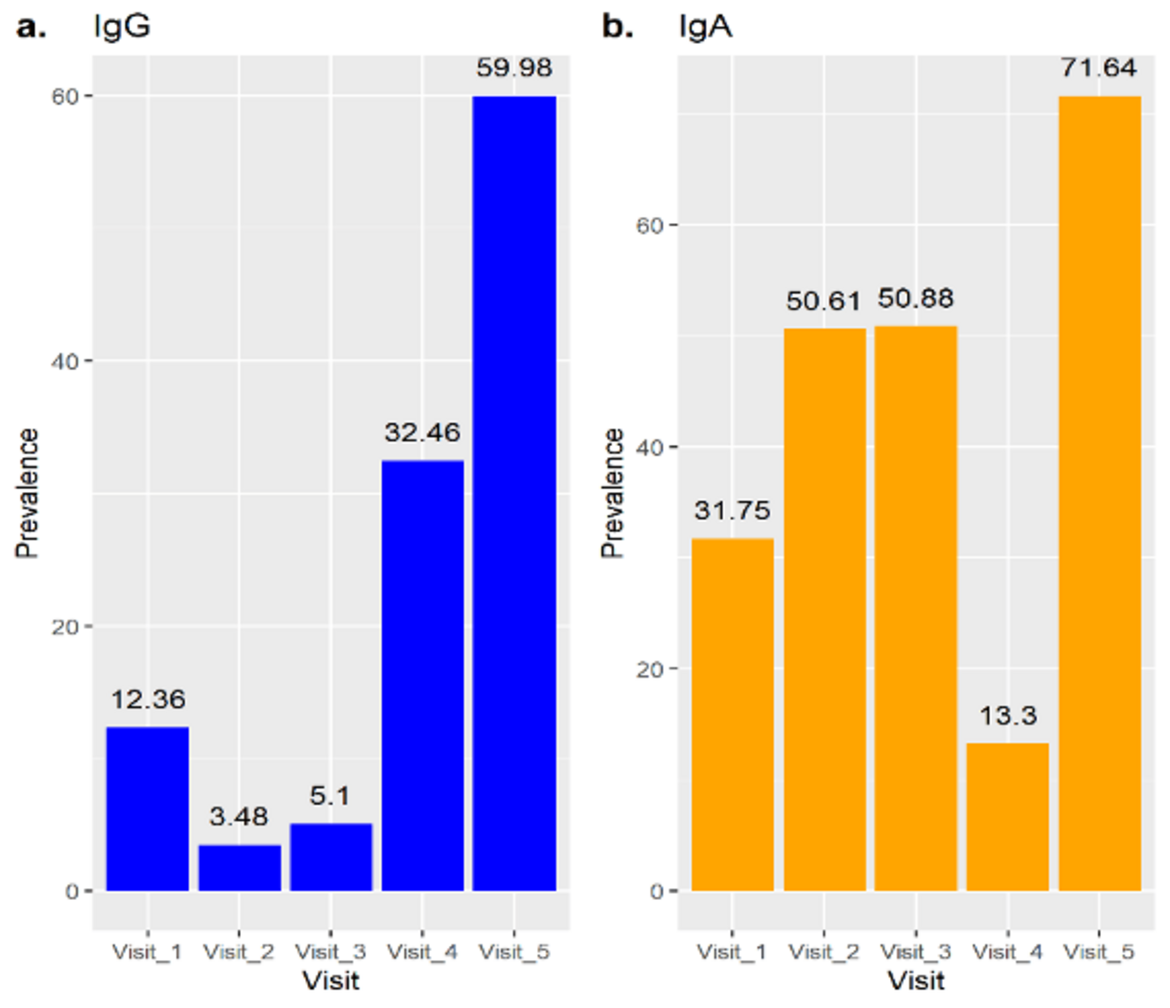
For those participants for who we were able to obtain a sample at every round, the progression of seroprevalence was from 12,36% to 78.86% from Round 1 to 5 (data not shown). In Figure 2 we show with boxplot graphics the evolution of IgG SARS-CoV-2 levels in the participants.
Figure 2: Box plot evolution of IgG Negative individuals at Visit 1
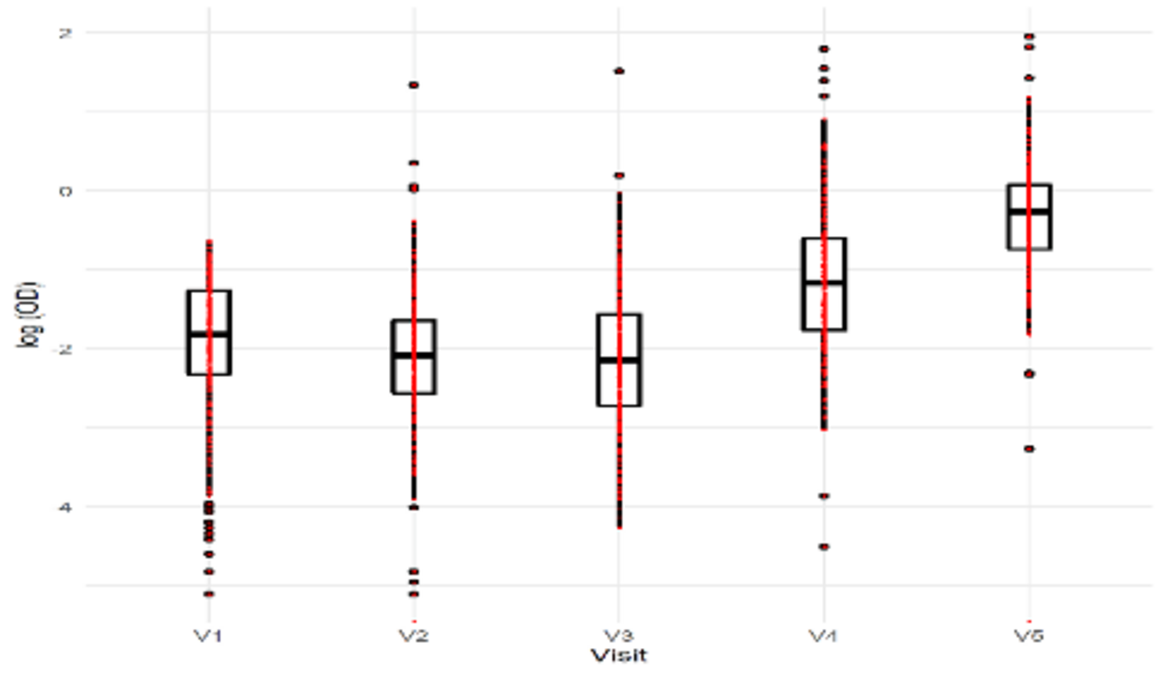
The analysis of IgG SARS-CoV-2 by household observed that while in the first three rounds, up to 50% of the households did not have SARS-CoV-2 infection, that trend changed over the 4th and 5th rounds when 75 per cent of the households had up to 25% and 50% SARS-CoV-2 infection, respectively. That shows the clear progression of the transmissions over the period studied, Figure 3.
Figure 3: IgG levels in between visits
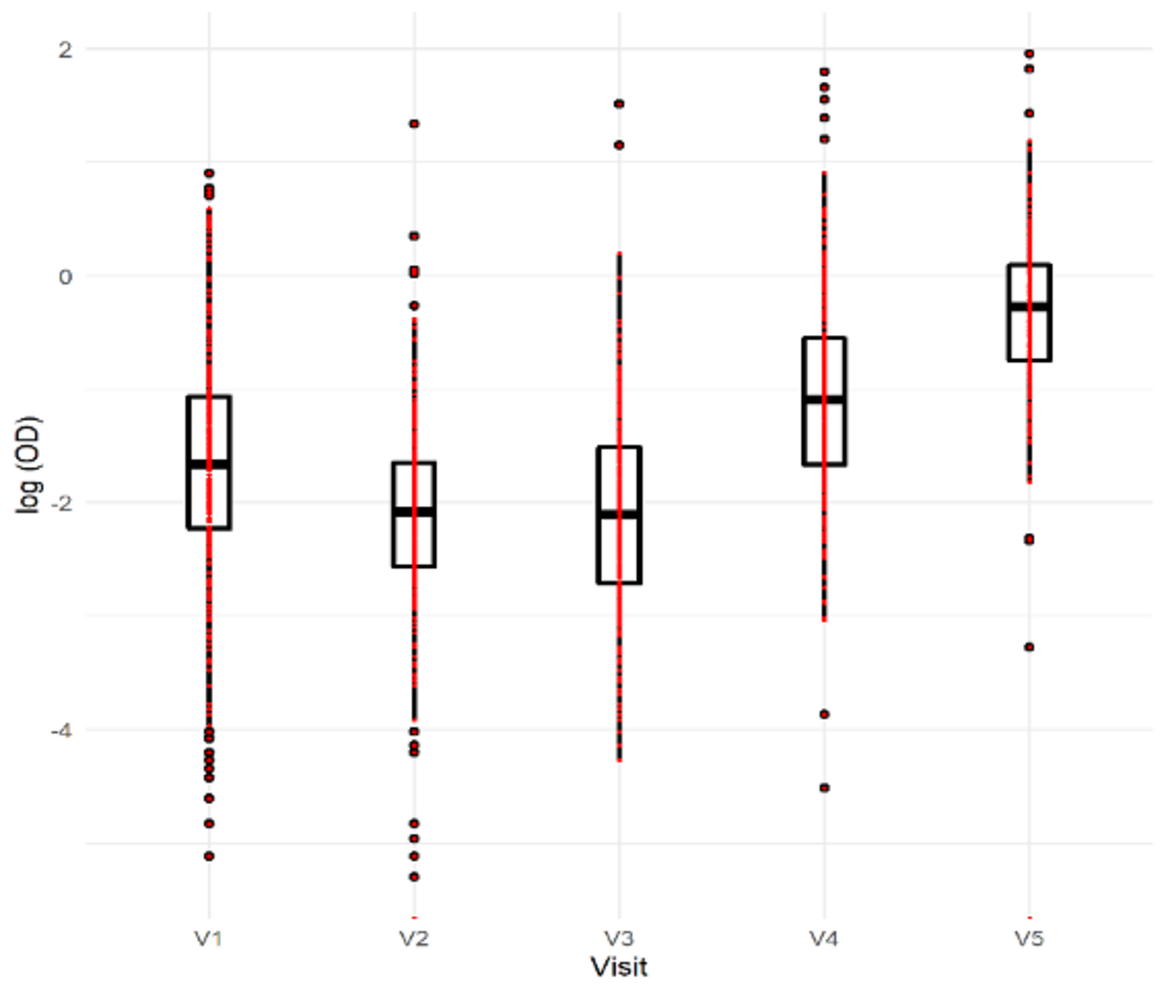
On the analysis of the differences in the results by dIgA and IgG for SARS-CoV-2 infection, we cannot find any correlation. This did not help to differentiate acute from past infections. This, and some discrepancies in the evolution of the results from the rounds, especially in rounds 2 and 3, is a limitation of our study. Interestingly, the quantitative responses on vaccine hesitancy in the serial-surveillance did not identify differences in the responses throughout the rounds. This shows that while we observed and increased household transmission on the studied community, this did not influence on the vaccine hesitancy of the participants.
In South Africa we conducted a household transmission study to define the epidemiological parameters of SARS-CoV-2 infection in a rural setting and assess demographic, behavioural and household-level risk factors for infection and transmission, and the feasibility of implementing preventive behaviours. The study site took place in the Thabong sub-district, Lejweleputswa, Free State Province from April to July 2021.
The study protocol was approved by the Human Research Ethics Council at Wits University and the Free State Province and district.
Using information provided daily from the local Department of Health, we identified adult (≥18 years) index COVID-19 cases with PCR confirmed SARS-CoV-2 infection and invited them to participate in the study, if eligible. We also enrolled their household members. Household visits were conducted at enrolment, and on day 7, 14 and 28, by a nurse and a fieldworker. Each participant completed a questionnaire and a daily paper-based symptom diary. Respiratory samples were collected for SARS-CoV-2 PCR (nasal swabs from those under 12 years and nasopharyngeal swabs from those older than 12), and paired with blood samples for SARS-CoV-2 IgG serology.
Twenty six index cases and 95 household contacts were enrolled. Three index cases and their households withdrew (n=12).
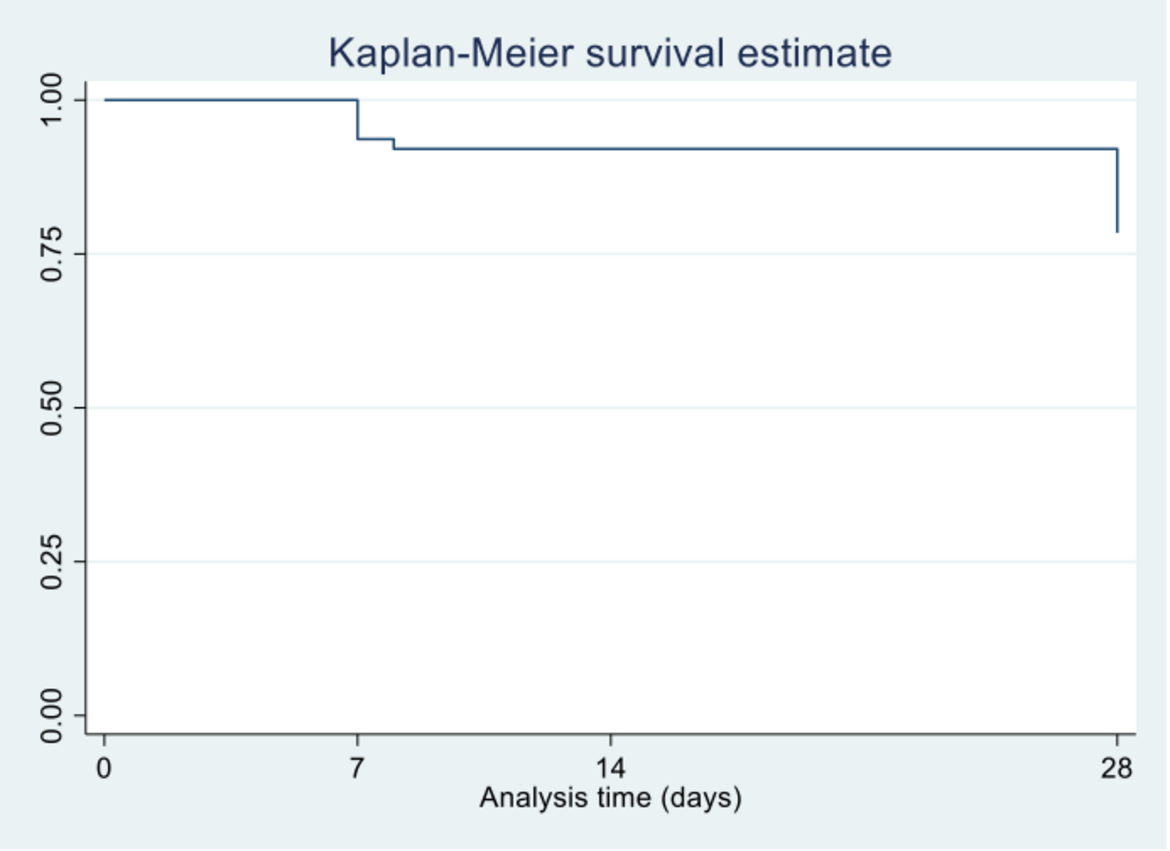
We found that of the 83 household contacts, 20 from 11 households had a positive SARS-CoV-2 PCR at enrolment. These were classified as co-primary cases. Of the 63 contacts who were SARS-CoV-2 PCR negative at enrolment, a further 5 became PCR positive during the 28-day follow-up. In addition, a further 5 household contacts had SARS-CoV-2 antibodies detected during the follow-up period. Cumulatively, 10 contacts (16%) had incident/new SARS-CoV-2 infection. These incident cases originated from 9 (39%) households. The incidence rate was estimated as 5.8 per 1,000 person-days (95%CI=3.14–11.95). Households with co-primary cases were associated with higher seroprevalence and incidence of SARS-CoV-2. Socio-demographic and health characteristics such as HIV or obesity were not associated with SARS-CoV-2 transmission and we did not identify any transmission risks inherent to a rural setting. This suggests that the approach to interventions to reduce the risk of transmission of COVID-19 in rural settings could be similar to those of non-rural settings. This study also suggests that symptom screening and SARS-CoV-2 PCR testing of household contacts through community outreach may not be a cost-effective strategy in case identification in future COVID-19 waves.
The manuscript for the study is being finalised and we expect to publish the findings in the BMJ Open journal.
Figure 1: SARS-CoV-2 PCR positivity rate from enrolment till 28 days of follow-up
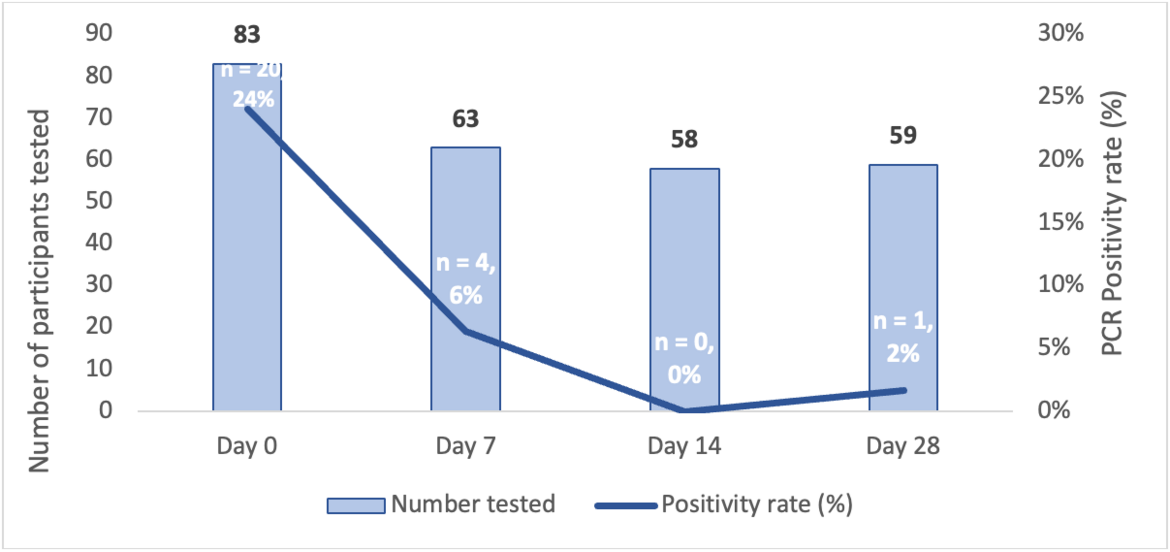
Figure 2: Kaplan-Meier survival curve showing time (in days) to incident cases
Duration: 20 months – completed 30.04.2022
Contact Person: Joy Mauti (joy.mauti(at)uni-heidelberg.de)
