- Sektion Nephrogenetik (AG Simons)
- Seniorprofessur Prof. Rappold
- Molekulare Neurogenetik (AG Berkel)
- Kardiogenetik (AG Hoffmann)
- Genomisches Neugeborenenscreening
- Translationale Neurowissenschaften (AG Althammer)
- Neurogastrogenetik (AG Mellein)
- Regulierung der Transkription bei Entwicklungsstörungen (AG Laugsch)
- Genetik neurologischer Entwicklungsstörungen (AG Schaaf)
- Mausmodelle für neurologische Entwicklungsstörungen (AG Fröhlich)
- Molekulare Humangenetik (AG Boutros)
- Forschung der genetischen Poliklinik
- Forschung in der Diagnostik
- Publikationen
Translationale Neurowissenschaften

Dr. rer. nat. Ferdinand Althammer
Schwerpunkt
Prader-Willi und Schaaf-Yang Syndrome, Oxytocin
The research group Translational Neuroscience (AG Althammer) explores the significance of oxytocin signaling in mouse and rat models of two genetic disorders: Prader-Willi (PWS) and Schaaf-Yang (SYS) syndromes. Prader-Willi Syndrome and Schaaf-Yang Syndrome are both rare genetic disorders that affect individuals from birth and have distinct clinical features and genetic causes:
Prader-Willi Syndrome (PWS)
Phenotype: PWS is characterized by a complex range of symptoms, including hypotonia (low muscle tone) in infancy, feeding difficulties, delayed development, intellectual disabilities, behavioral problems, and a compulsive overeating and obesity tendency, which can lead to life-threatening health issues if not managed.
Underlying Genetic Cause: PWS is primarily caused by a lack of expression of certain genes on the paternal chromosome 15, typically due to a deletion on the paternal chromosome 15 (del15q11-q13) or uniparental disomy (both copies of chromosome 15 inherited from the mother). This genetic abnormality results in the absence of crucial genes in the brain, leading to the hallmark symptoms of PWS.
Schaaf-Yang Syndrome (SYS)
Phenotype: SYS shares some similarities with PWS, such as intellectual disabilities and hypotonia. However, it is distinguished by distinct features like joint contractures, involuntary movements, and distinctive facial features. Individuals with SYS may also have autism spectrum disorders and sleep disturbances.
Underlying Genetic Cause: SYS is primarily caused by mutations in the MAGEL2 gene, which is located on chromosome 15. This gene is typically paternally imprinted, and mutations can disrupt normal neurodevelopment, resulting in the observed clinical features.
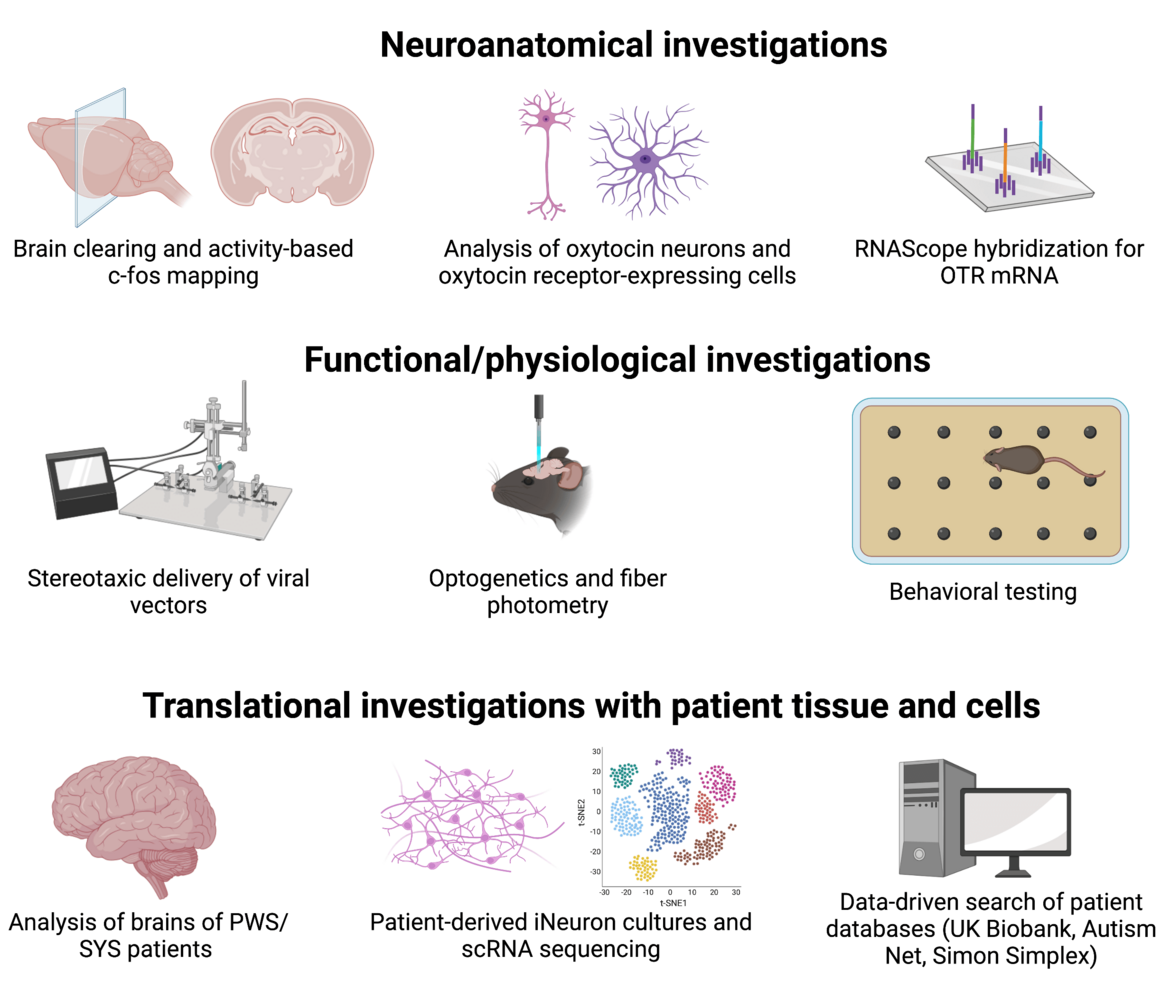
Our investigations extend beyond animal models, as we also utilize patient-derived neuronal cultures and post-mortem brain tissue from individuals with Prader-Willi syndrome. Employing a diverse array of techniques, including anatomical, physiological, functional, and genetic tools, we aim to unravel the intricate cellular and behavioral implications associated with Prader-Willi and Schaaf-Yang syndromes. Our ultimate objective is to obtain a comprehensive mechanistic understanding of oxytocinergic signaling in these conditions, thereby establishing a solid groundwork for the development of tailored treatment options for each patient. Through our translational investigations, we aspire to make meaningful contributions towards enhancing patient care and well-being.
Techniques and methodologies in our lab comprise:
- Immunohistochemistry
- Three-dimensional reconstruction and analysis via the Imaris software
- RNAScope in situ hybridization
- Brain region-specific viral vector-based expression of proteins
- In vivo optogenetics and imaging
- Behavioral experiments
- DISCO tissue clearing
- Brain-wide activity mapping via ClearMap
- Patient-derived cell culture
- Analysis of post-mortem brain tissue
- Proteomics
- Sektion Nephrogenetik (AG Simons)
- Seniorprofessur Prof. Rappold
- Molekulare Neurogenetik (AG Berkel)
- Kardiogenetik (AG Hoffmann)
- Genomisches Neugeborenenscreening
- Translationale Neurowissenschaften (AG Althammer)
- Neurogastrogenetik (AG Mellein)
- Regulierung der Transkription bei Entwicklungsstörungen (AG Laugsch)
- Genetik neurologischer Entwicklungsstörungen (AG Schaaf)
- Mausmodelle für neurologische Entwicklungsstörungen (AG Fröhlich)
- Molekulare Humangenetik (AG Boutros)
- Forschung der genetischen Poliklinik
- Forschung in der Diagnostik
- Publikationen




