EXPERIMENTAL NEUROONCOLOGY
OVERVIEW
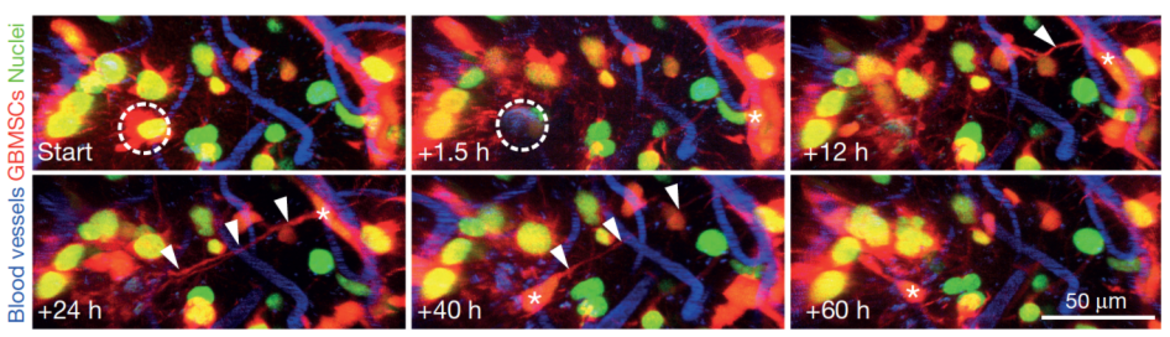
The central interest of the research group is to understand how primary and metastatic tumors progress in the brain, and how this information can be used to improve therapies. The research focus lies on clinically relevant, but also basic questions in brain tumor research, but also other neurological diseases. To study the initiation, progression and therapeutic responses of diseases of the central nervous system, we have established refined animal models using in vivo two-photon microscopy in our DKFZ lab. This methodology allows studying brain cancer cell populations and their dynamic behavior over many months, including their cellular components, gene expression, blood vessels, glia cells, neurons, intercellular communications, and important physiological and therapeutical parameters like hypoxia, blood flow velocity, and vascular permeability. This unique approach makes it possible to investigate dynamic interactions of cells, and the key mechanisms in a live organism over long periods of time in high resolution. We currently pursue the following main research projects:
The role of tumor microtubes in brain tumor progression: We discovered that ultra-long and ultra-thin membrane extensions of astrocytoma (including glioblastoma) cells are highly relevant for tumor progression and resistance to therapies (Osswald Nature 2015). The resulting multicellular tumor network allows intensive intercellular communication, and better cellular homeostasis, which results in resistance to radiotherapy. In ongoing projects, we aim to better understand 1) whether tumor microtubes are also relevant for communication with cell types of the normal brain; 2) how tumor microtubes, and the functional network they form, can be optimally targeted by novel therapies – to break the notorious treatment resistance of many brain tumors; 3) whether and how epilepsy, a frequent problem for brain tumor patients, is related to these discoveries.
The role of neuron-tumor synapses for brain tumor progression: We discovered that neurons form glutamatergic synapses with tumor cells of incurable gliomas (Venkataramani Nature 2019). These malignant synapses are fully functional, and stimulate brain tumor networks, leading to proliferation and brain invasion. These discoveries shed a new light on tumor-related epilepsy, and the vicious circle between epileptic seizures and brain tumor growth, but also point towards novel avenues to tackle brain tumor growth in the future: by inhibiting neuron-glioma synapses with specific drugs, some of them already clinically established for other indications.
https://www.dkfz.de/en/neuroonkologie/AG_Winkler.html
Brain metastases prevention: This novel approach, supported by results from own previous work (Kienast Nat Med 2010; Ilhan-Mutlu Mol Cancer Ther 2016), appears to be a clinically feasible concept for certain tumor entities. Prof. Winkler is Principal Investigator of the prevent_BM consortium, funded by the German Cancer Aid (Deutsche Krebshilfe), which consequently explores this exciting translational research concept as a novel strategy in Oncology.
Team
Arbeitsgruppenleiter
Wiss. Mitarbeiter/-innen
-

Manuel Feinauer
-
Dr. med. Erik Jung
Schwerpunkt
Parkinson, Seltene Neurogenetische Erkrankungen, Hereditäre Spastische Spinalparalysen (HSP) und andere Bewegungsstörungen
-

Dr. Matthia Karreman
-

Miriam Ratliff
SELECTED PUBLICATIONS
- Winkler F, Wick W. Harmful networks in the brain and beyond. Science. 2018 Mar 9;359(6380):1100-1101
- Weil S, Osswald M, Solecki G, Grosch J, Jung E, Lemke D, Ratliff M, Hänggi D, Wick W, Winkler F (2017). Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro Oncol. 2017 Oct 1;19(10):1316-1326
- Jung E, Osswald M, Blaes J, Wiestler B, Sahm F, Schmenger T, Solecki G, Deumelandt K, Kurz FT, Xie R, Weil S, Heil O, Thomé C, Gömmel M, Syed M, Häring P, Huber PE, Heiland S, Platten M, von Deimling A, Wick W, Winkler F. Tweety-Homolog 1 Drives Brain Colonization of Gliomas. J Neurosci. 2017 Jul 19;37(29):6837-6850
- Osswald M, Blaes J, Liao Y, Solecki G, Gömmel M, Berghoff AS, Salphati L, Wallin JJ, Phillips HS, Wick W, Winkler F. Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin Cancer Res. 2016 Dec 15;22(24):6078-6087
- Ilhan-Mutlu A, Osswald M, Liao Y, Goemmel M, Reck M, Miles D, Mariani P, Gianni L, Lutiger B, Nendel V, Strock S, Perez-Moreno PD, Thorsen F, von Baumgarten LD, Preusser M, Wick W, Winkler F. Bevacizumab prevents brain metastases formation in lung adenocarcinoma. Mol Cancer Ther. 2016 Apr;15(4):702-10
- Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, Weil S, Horstmann H, Wiestler B, Syed M, Huang L, Ratliff M, Karimian Jazi K, Kurz FT, Schmenger T, Lemke D, Gommel M, Pauli M, Liao Y, Haring P, Pusch S, Herl V, Steinhauser C, Krunic D, Jarahian M, Miletic H, Berghoff AS, Griesbeck O, Kalamakis G, Garaschuk O, Preusser M, Weiss S, Liu H, Heiland S, Platten M, Huber PE, Kuner T, von Deimling A, Wick W, Winkler F. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015 Dec 3;528(7580):93-8
- von Baumgarten L, Brucker D, Tirniceru A, Kienast Y, Grau S, Burgold S, Herms J, Winkler F. Bevacizumab has differential and dose-dependent effects on glioma blood vessels and tumor cells. Clin Cancer Res. 2011 Oct 1;17(19):6192-205
- Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert W, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010 Jan;16(1):116-22
- Winkler F, Kozin SV, Tong RT, Chae S, Booth MF, Garkavtsev I, Xu L, Hicklin DK, Fukumura D, di Tomaso E, Munn LL, RK Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, Angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004 Dec;6(6):553-63










